
The main goal of our research is to understand the mechanisms of the photosynthetic machinery in different organisms and at different levels of the structural and functional complexities –with special attention to the interplay between the dynamic features and the regulation of excitation energy and electron flow. We also design and construct innovative scientific instruments.
By using mainly circular dichroism (CD) spectroscopy, we have established that the protein complexes in the chloroplast thylakoid membranes of higher plants and many algae are assembled into extended arrays with long-range chiral order, which are capable of undergoing sizeable reversible reorganizations upon changes in the environmental conditions (Garab 2014; Lambrev and Akhtar 2019). The macrodomain formation explains the sorting of LHCII:PSII and LHCI:PSI supercomplexes and the stacking of membranes, the basis of the self-assembly of granal ultrastructure (Mustárdy and Garab 2003). Using electron tomography, we revealed the quasi-helical organization of the granum-stroma thylakoid membrane assembly (Mustárdy et al. 2008).
The anisotropic CD (ACD) of macroscopically aligned samples – an extension of the CD spectroscopy – provides additional information about the molecular orientation of the participating pigment dipoles. The plant light-harvesting complex II (LHCII) in isolated form or in the native membrane has a distinct ACD signature that, which provides geometric constraints helping to identify excitonic states shared between specific chlorophylls (Akhtar et al. 2019).
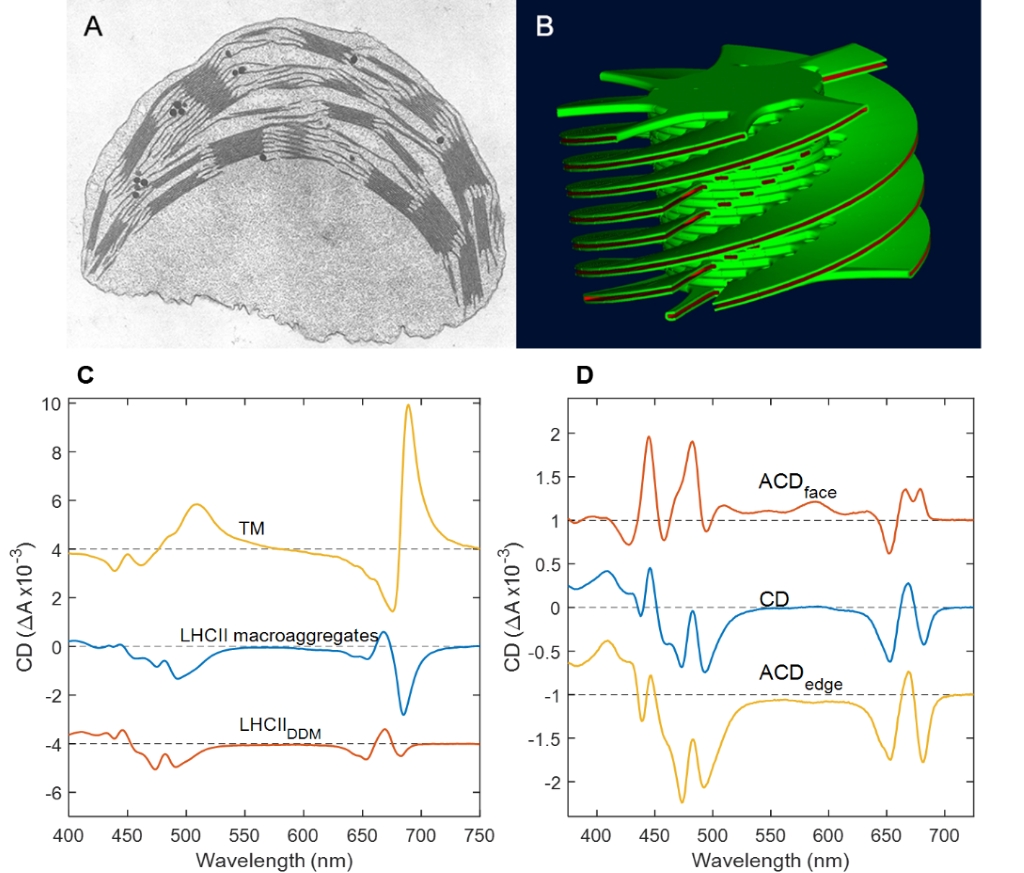
Macroorganization of the thylakoid membrane: A. Thin-section electron micrograph of higher-plant chloroplast. B. 3D computer model of the granal thylakoid membrane based on serial-sectioning electron micrographs. C. Psi-type CD spectra of pea chloroplasts, lamellar macroaggregates of LHCII and the excitonic CD spectrum of detergent-solubilized LHCII. D. ACD spectra of LHCII in oriented lipid bilayers (Mustárdy and Garab 2003, Lambrev and Akhtar 2019).
By using the technique of small-angle neutron scattering (SANS) in vitro and in vivo under a large variety of physiologically important conditions and in different mutants, we have also shown that the thylakoid membranes should not be portrayed as simply providing a scaffold for the photosynthetic functions but, by fine-tuning their multilamellar 3D ultrastructure, they actively participate in different regulatory mechanisms (Ünnep et al. 2014)). The non-invasive techniques of CD and SANS are routinely used towards the better understanding of the nature and physiological significance of structural dynamics of thylakoid membranes on the mesoscopic size scale.
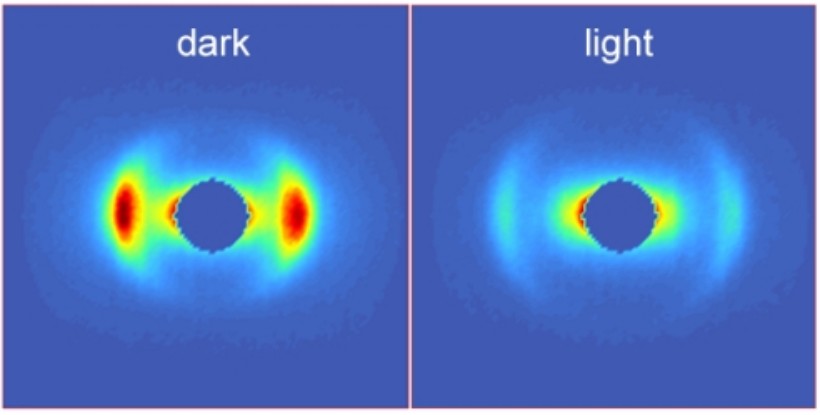
Small-angle neutron scattering profiles of thylakoid membranes in the dark or after illumination.
Akhtar, P., Lindorfer, D., Lingvay, M., Pawlak, K., Zsiros, O., Siligardi, G., et al. (2019) Anisotropic circular dichroism of light-harvesting complex II in oriented lipid bilayers: Theory meets experiment. J. Phys. Chem. B 123: 1090–1098.
Garab, G. (2014) Hierarchical organization and structural flexibility of thylakoid membranes. Biochim Biophys Acta 1837: 481–494.
Lambrev, P.H. and Akhtar, P. (2019) Macroorganisation and flexibility of thylakoid membranes. Biochemical Journal 476: 2981–3018.
Lambrev, P.H., Akhtar, P. and Tan, H.-S. (2020) Insights into the mechanisms and dynamics of energy transfer in plant light-harvesting complexes from two-dimensional electronic spectroscopy. Biochim. Biophys. Acta 1861: 148050.
Mustárdy, L., Buttle, K., Steinbach, G. and Garab, G. (2008) The three-dimensional network of the thylakoid membranes in plants: quasihelical model of the granum-stroma assembly. Plant Cell 20: 2552–2557.
Mustárdy, L. and Garab, G. (2003) Granum revisited. A three-dimensional model – where things fall into place. Trends Plant Sci. 8: 117–122.
Ünnep, R., Zsiros, O., Solymosi, K., Kovács, L., Lambrev, P.H., Tóth, T., et al. (2014) The ultrastructure and flexibility of thylakoid membranes in leaves and isolated chloroplasts as revealed by small-angle neutron scattering. Biochim. Biophys. Acta. 1837: 1572–1580.
We study the elementary mechanisms, pathways and kinetics of the processes of light absorption, migration of the excitation energy and photochemical charge separation in the photosynthetic apparatus. We apply a variety of optical spectroscopy techniques to study these processes in vivo and in vitro – using isolated pigment-protein complexes, native or reconstituted membranes. One of the most capable techniques to probe the ultrafast excitation dynamics is multidimensional electronic spectroscopy. We have been working in close collaboration with the group of Howe-Siang Tan at NTU, Singapore applying multidimensional electronic spectroscopy to reveal new details on the mechanisms and dynamics of photosynthetic light harvesting (Lambrev, Akhtar and Tan 2020). By two-dimensional electronic spectroscopy we could follow simultaneously uphill and downhill energy transfer in light-harvesting complex II (Akhtar et al. 2017) and in Photosystem I (Akhtar et al. 2018). A clear temperature dependence of uphill energy transfer processes was also discerned, consistent with the detailed-balance condition (Akhtar et al. 2019); these uphill pathways ensure fast and efficient energy transfer at physiological temperatures (Do et al. 2019).
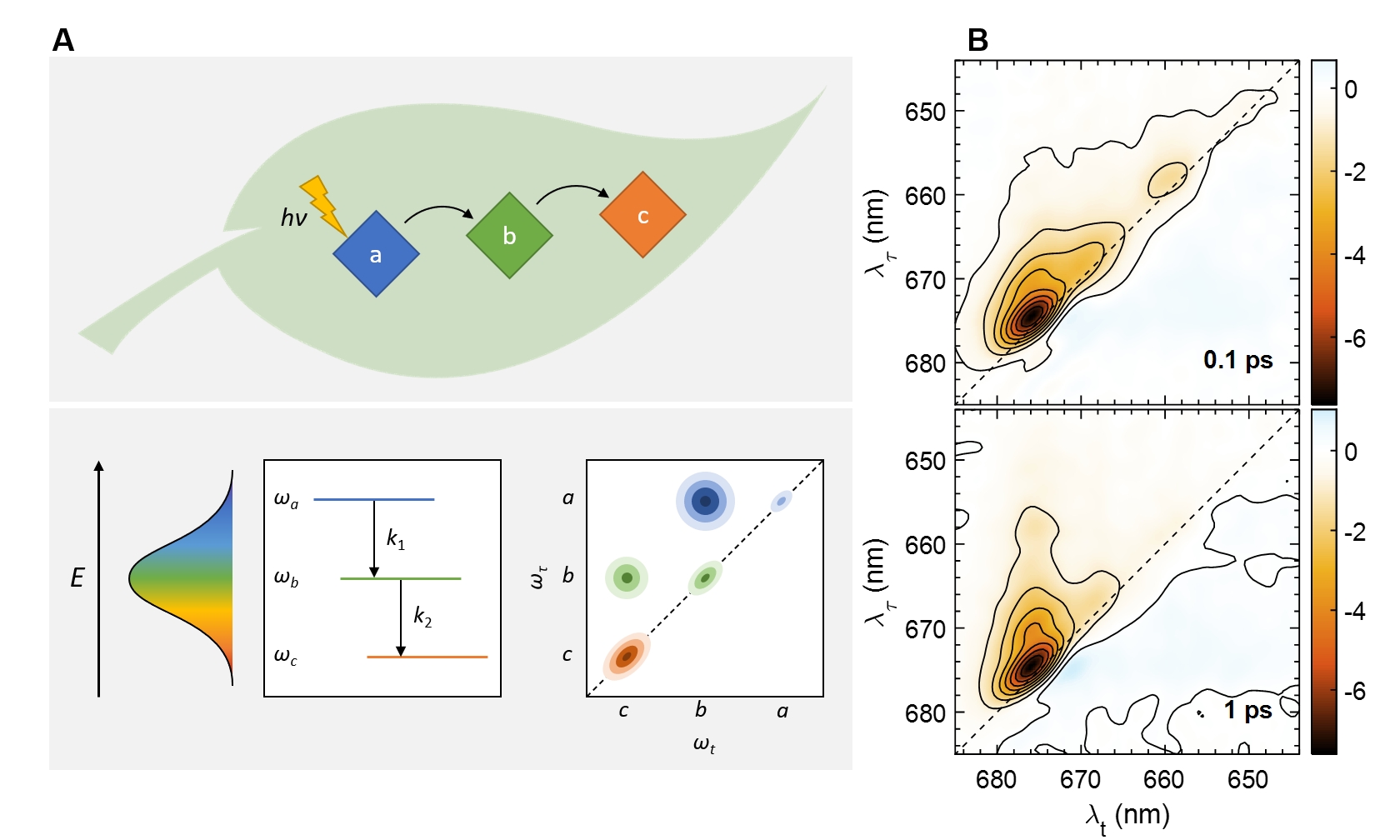
Probing energy transfer in photosynthetic complexes by two-dimensional electronic spectroscopy. A. Relaxation between coupled excitonic transitions appears is visualized as dynamic cross-peaks in the 2D electronic spectra (Lambrev et al. 2020). B. 2D electronic spectra of LHCII from Bryopsis corticulans at 77 K at two different waiting (delay) times (Akhtar and Lambrev 2020).
By using two-dimensional electronic spectroscopy in combination with structure-based theoretical modelling, we could follow the dynamics of energy transfer and charge separation in cyanobacterial Photosystem I complexes at 77 K and determine that primary charge separation upon direct excitation of the reaction centre occurs in about 1 ps (Akhtar et al. 2021).
Akhtar, P., Caspy, I., Nowakowski, P.J., Malavath, T., Nelson, N., Tan, H.-S. and Lambrev, P.H. (2021) Two-dimensional electronic spectroscopy of a minimal Photosystem I complex reveals the rate of primary charge separation. Journal of the American Chemical Society 143: 14601–14612.
Akhtar, P., Do, T.N., Nowakowski, P.J., Huerta-Viga, A., Khyasudeen, M.F., Lambrev, P.H. and Tan, H.-S. (2019) Temperature dependence of the energy transfer in LHCII revealed by two-dimensional electronic spectroscopy. J. Phys. Chem. B 123: 6765–6775.
Akhtar, P. and Lambrev, P.H. (2020) On the spectral properties and excitation dynamics of long-wavelength chlorophylls in higher-plant photosystem I. Biochim. Biophys. Acta 1861: 148274.
Akhtar, P., Zhang, C., Do, T.N., Garab, G., Lambrev, P.H. and Tan, H.-S. (2017) Two-dimensional spectroscopy of chlorophyll a excited-state equilibration in light-harvesting complex II. J. Phys. Chem. Lett. 8: 257–263.
Akhtar, P., Zhang, C., Liu, Z., Tan, H.-S. and Lambrev, P.H. (2018) Excitation transfer and trapping kinetics in plant photosystem I probed by two-dimensional electronic spectroscopy. Photosynth. Res. 135: 239–250.
Do, T.N., Huerta-Viga, A., Akhtar, P., Nguyen, H.L., Nowakowski, P.J., Khyasudeen, M.F., et al. (2019) Revealing the excitation energy transfer network of Light-Harvesting Complex II by a phenomenological analysis of two-dimensional electronic spectra at 77 K. J. Chem. Phys. 151: 205101.
Lambrev, P.H., Akhtar, P. and Tan, H.-S. (2020) Insights into the mechanisms and dynamics of energy transfer in plant light-harvesting complexes from two-dimensional electronic spectroscopy. Biochim. Biophys. Acta 1861: 148050.
By using CD spectroscopy, we have revealed that the pigment-pigment interactions in LHCII are sensitive to its molecular environment (Lambrev et al. 2007, Akhtar et al. 2015). Extracting of the complexes from the membrane and solubilization with detergents perturbs the native structure. If not bound to the PSII supercomplexes, LHCII units tend to associate into energetically connected domains (Lambrev et al. 2011). Macrostructural rearrangements of a similar kind involve other antenna complexes of the LHC superfamily, such as FCP in diatoms Ghazaryan 2016 (Ghazaryan et al. 2016). In model membranes, LHCII forms packed clusters (Tutkus et al. 2018; Tutkus et al. 2021), where the excited-state lifetime is inversely correlated to the protein density (Akhtar et al. 2019). This type of excitation quenching is accompanied by spectroscopic signatures similar to the photoprotective non-photochemical quenching in vivo, including the formation of chlorophyll-chlorophyll charge-transfer states that can effectively dissipate the excitation energy (Ostroumov et al. 2020).
Lambrev, P., Várkonyi, Z., Krumova, S., Kovács, L., Miloslavina, Y., Holzwarth, A. R., and Garab, G. (2007) Importance of trimer–trimer interactions for the native state of the plant light-harvesting complex II. Biochim. Biophys. Acta - Bioenergetics 1767:847–853.
Akhtar, P., Dorogi, M., Pawlak, K., Kovács, L., Bóta, A., Kiss, T., Garab, G., and Lambrev, P.H. (2015) Pigment interactions in light-harvesting complex II in different molecular environments. J. Biol. Chem. 290:4877–4886.
Lambrev, P.H., Schmitt, F. J., Kussin, S., Schoengen, M., Várkonyi, Z., Eichler, H. J., Garab, G., and Renger, G. (2011) Functional domain size in aggregates of light-harvesting complex II and thylakoid membranes. Biochim. Biophys. Acta 1807:1022–1031.
Ghazaryan, A., Akhtar, P., Garab, G., Lambrev, P. H., and Buchel, C. (2016) Involvement of the Lhcx protein Fcp6 of the diatom Cyclotella meneghiniana in the macro-organisation and structural flexibility of thylakoid membranes. Biochim. Biophys. Acta - Bioenergetics 1857: 1373–1379.
Tutkus, M., Akhtar, P., Chmeliov, J., Görföl, F., Trinkunas, G., Lambrev, P. H., and Valkunas, L. (2018) Fluorescence microscopy of single liposomes with incorporated pigment–proteins. Langmuir 34:14410–14418.
Tutkus, M., Chmeliov, J., Trinkunas, G., Akhtar, P., Lambrev, P. H., and Valkunas, L. (2021) Aggregation-related quenching of LHCII fluorescence in liposomes revealed by single-molecule spectroscopy. J. Photoch. Photobio. B 218:112174.
Akhtar, P., Görföl, F., Garab, G., and Lambrev, P. H. (2019) Dependence of chlorophyll fluorescence quenching on the lipid-to-protein ratio in reconstituted light-harvesting complex II membranes containing lipid labels. Chem. Phys. 522:242–248.
Ostroumov, E. E., Götze, J. P., Reus, M., Lambrev, P. H., and Holzwarth, A. R. (2020) Characterization of fluorescent chlorophyll charge-transfer states as intermediates in the excited state quenching of light-harvesting complex II. Phot. Res. 144:171–193.
Our Dynamic Exchange Model (DEM) extends the ‘standard’ fluid-mosaic bilayer membrane model and provides a framework to explain the thoroughly documented polymorphic phase behaviour and structural dynamics of plant thylakoid membranes (TMs). According to DEM: (i) non-bilayer lipids are ‘forced’ to enter the bilayer by membrane-intrinsic proteins; but (ii) lipids, due to their high non-bilayer propensity, can segregate out from the membrane; (iii) the lumenal and stroma-side aqueous phases, which are also densely packed by proteins, contain lipocalins and lipocalin-like proteins that are capable of binding lipid molecules; and (iv) non-bilayer lipids and non-lamellar lipid phases play key roles in the self-assembly and reorganizations of the vesicular membrane network via assisting membrane fusions and junctions. By this means, non-bilayer lipids are proposed to self-regulate the membrane homeostasis, safe-guarding the remarkably high protein-to-lipid ratio of TMs and lend them high structural plasticity. In accordance with our earlier hypothesis, our recent experiments have revealed that the non-bilayer phases are found outside the domains containing the protein-rich regions of TMs, i.e., the region of bilayer-embedded supercomplexes of the photosynthetic machinery. We have demonstrated the participation of an enhanced isotropic phase in activating the photoprotective lumenal enzyme (VDE). Non-bilayer lipid phases might also be given rise by lipid molecules associated with different water-soluble lipocalin or lipocalin-like proteins. We have also shown that (an) isotropic lipid phase(s) participate(s) in the fusion of TMs. The inverted hexagonal (HII) phase appears to originate from lipids encapsulating stroma-exposed proteins or polypeptides. The nature and molecular mechanisms of the formation of different non-bilayer lipid phases and their roles in regulatory processes and in the energization of TMs are investigated.
|
|
|
Lipid polymorphysm of plant TMs
Dlouhy, O., Karlicky, V., Javornik, U., Kurasova, I., Zsiros, O., Sket, P., et al. (2022) Lipid polymorphism of plant thylakoid membranes. Structural and functional units associated with non-bilayer phases. Biochim. Biophys. Acta 1863: 83-83.
Dlouhý, O., Kurasová, I., Karlický, V., Javornik, U., Šket, P., Petrova, N.Z., et al. (2020) Modulation of non-bilayer lipid phases and the structure and functions of thylakoid membranes: effects on the water-soluble enzyme violaxanthin de-epoxidase. Sci. Rep. 10: 11959.
Garab, G., Ughy, B., de Waard, P., Akhtar, P., Javornik, U., Kotakis, C., et al. (2017) Lipid polymorphism in chloroplast thylakoid membranes–as revealed by 31P-NMR and time-resolved merocyanine fluorescence spectroscopy. Sci. Rep. 7: 13343.
Garab, G., Yaguzhinsky, L.S., Dlouhý, O., Nesterov, S.V., Špunda, V. and Gasanoff, E.S. (2022) Structural and functional roles of non-bilayer lipid phases of chloroplast thylakoid membranes and mitochondrial inner membranes. Prog. Lipid Res. 86: 101163.
Photosystem II (PSII) uses light energy to oxidize water, providing us with an oxygenic atmosphere. Via splitting water, PSII is the ultimate source of virtually all reducing equivalents in the Biosphere. Its activity is routinely monitored by recording the variable chlorophyll-a fluorescence (ChlF). The ‘mainstream’ interpretation of ChlF, which has never been free of controversies, is based on the 1963 two-state model, PSIIO, open state and PSIIC closed state, containing QA, the first stable quinone acceptor, in oxidized state and reduced state, respectively. Recently, we discovered rate-limiting steps in the dark-to-light transition of PSII and revealed the gradual formation of PSIIL, the light-adapted closed state, with stabilized charges compared to PSIIC. We also showed that the physical mechanism of ChlF must be laid on new foundations, in which intense steady-state and transient local electric fields and dielectric relaxation processes and the lipid matrix of the RCs and protein memory effects play key roles – these are being studied by advanced laser spectroscopy, structural and molecular biology techniques. The results will most certainly lead to a deeper understanding of the physical and molecular mechanisms of the primary processes of photosynthesis and may aid the design of artificial light-energy converting molecular devices.
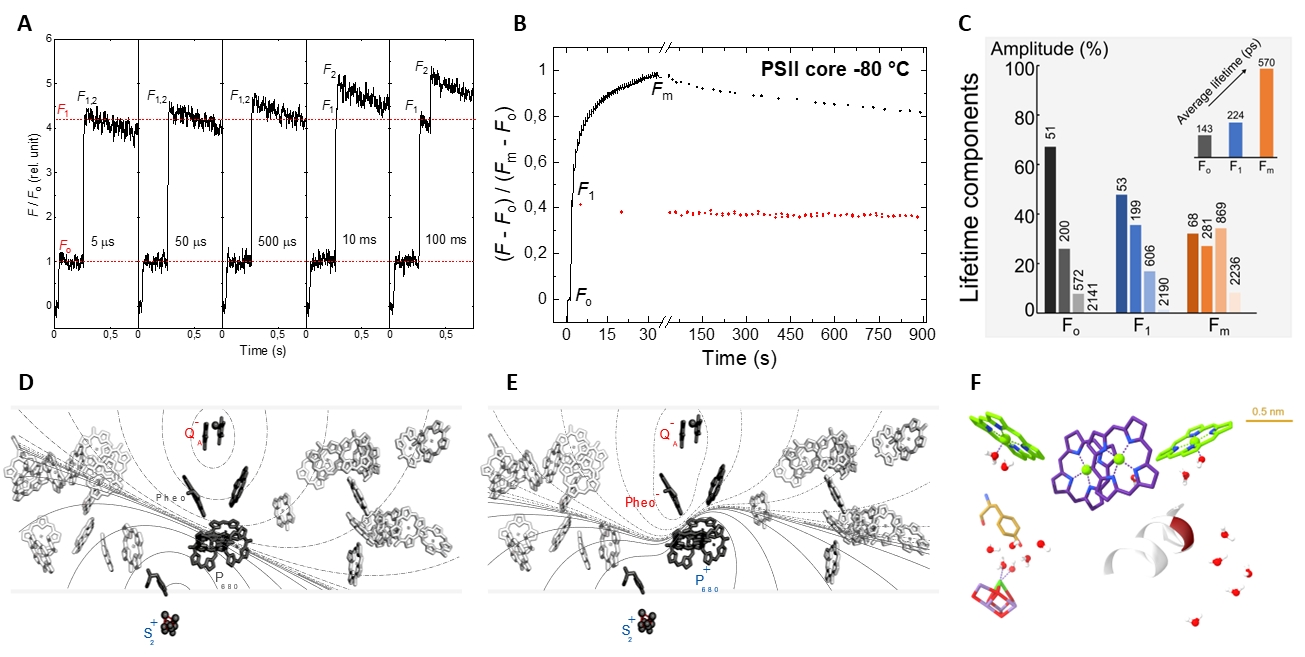
The main novel features of the dark-to-light transition of PSII and the physical mechanism of ChlF. (A) Rate limiting steps observed upon a double-flash excitation of PSII; (B) the gradual transition of PSIIO–PSIIC–PSIIL, as reflected by the Fo–F1–Fm transitions of ChlF, and relaxations at a cryogen temperature; (C) fluorescence lifetime components of PSII; (D and E) steady-state and transient electric fields; and (F) regions of high polarizability in PSII.
Garab, G., Magyar, M., Sipka, G. and Lambrev, P.H. (2023) Chlorophyll-a fluorescence induction on new grounds: quantum efficiency versus the light-adapted state of photosystem II. J. Exp. Bot. doi:10.1093/jxb/erad252
Magyar, M., Akhtar, P., Sipka, G., Han, W., Li, X., Han, G., et al. (2022) Dependence of the rate-limiting steps in the dark-to-light transition of photosystem II on the lipidic environment of the reaction center. Photosynthetica 60: 147-156.
Magyar, M., Sipka, G., Han, W., Li, X., Han, G., Shen, J.-R., et al. (2023) Characterization of the Rate-Limiting Steps in the Dark-To-Light Transitions of Closed Photosystem II: Temperature Dependence and Invariance of Waiting Times during Multiple Light Reactions. International Journal of Molecular Sciences 24: 94.
Magyar, M., Sipka, G., Kovács, L., Ughy, B., Zhu, Q., Han, G., et al. (2018) Rate-limiting steps in the dark-to-light transition of Photosystem II-revealed by chlorophyll-a fluorescence induction. Sci. Rep. 8: 2755.
Sipka, G., Magyar, M., Mezzetti, A., Akhtar, P., Zhu, Q., Xiao, Y., et al. (2021) Light-Adapted Charge-Separated State of Photosystem II: Structural and Functional Dynamics of the Closed Reaction Center. Plant Cell 33: 1286–1302.
Sipka, G., Nagy, L., Magyar, M., Akhtar, P., Shen, J.-R., Holzwarth, A.R., et al. (2022) Light-induced reversible reorganizations in closed Type II reaction centre complexes: physiological roles and physical mechanisms. Open Biology 12: 220297.
Microalgae are worth investigating from two aspects, one as a basic research model and the other as a subject for industrial applications, both complement each other. The cell division and stress adaptation processes of microalgae are in the focus, which are the basis for high biomass yield and production of bioactive compounds. We examine microalgae on gene level, protein level and on supra-individual level and study the membrane structure and function. Our research so far shows that a single-cell population can be described as a virtual tissue, where the cells that constitute the population are at different levels of differentiation.We have shown that population growth is inevitably arrested; the stop is not a consequence of starvation or cell contact inhibition, just as the decrement of colony-forming unit (CFU), they are genetically programmed (Ughy et al. 2023). The cells of a population in a stationary state will only commence dividing above a certain dilution (minimal stationary cell concentration, MSCC). This property is unversal among the unicellular organisms and has important medical and biotechnological implications (Ughy et al. 2023). We investigate the structure and function of the membrane (Kóbori et al. 2018). We employ commercially available and proprietary microalgal strains isolated from natural sources. We are involved in projects related to the biotechnological use of selected algal cultures, investigating the use of algae in animal feed and the secondary metabolites of algae in the health care. In laboratory conditions, we optimise the parameters that influence microalgal growth. Microalgae often produce increased amounts of bioactive compounds under stress conditions; we are going to investigate the relationship between stress adaptation and bioactive production (Kanna et al. 2021). We are examining the basic molecular processes of individual cell division with genetical approach. We try to explain the development of the different growth phases of cell cultures with molecular mechanisms of cells, i.e. we will search for links between single cell division and population growth. Our investigations include the study of microalgal growth parameters, photosynthetic processes, fatty acid and protein composition. We are trying to establish self-sustaining bacterial consortia, and with this knowledge we aim to conserve and improve soil stability and enhance crop production.
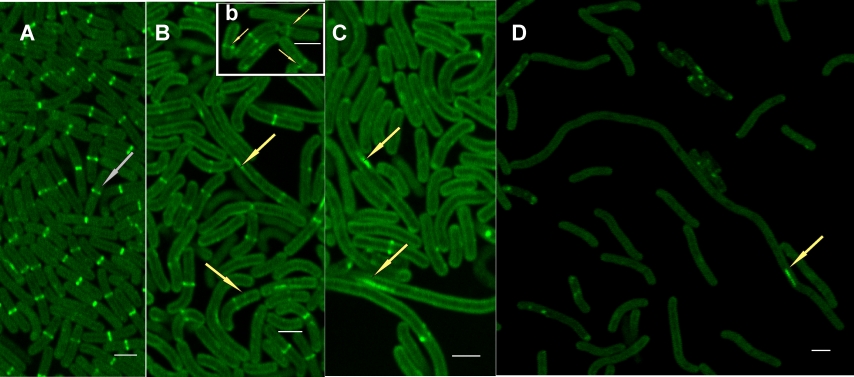
CLSM image of EGFP signals in Synechococcus phosphatidylglicerol (PG) synthesis mutant live cells grown for 4 days in the presence of PG (A) and during PG depletion on the 4th day (B, b) 8th day (C) and 12th day (D)of the depletion process. (Kóbori et al. 2018)
Ughy, B., Nagyapati, S., Lajko, D. B., Letoha, T., Prohaszka, A., Deeb, D., Der, A., Pettko-Szandtner, A., and Szilak, L. (2023) Reconsidering Dogmas about the Growth of Bacterial Populations. Cells-Basel 12: 1430.
Kóbori, T. O., Uzumaki, T., Kis, M., Kovács, L., Domonkos, I., Itoh, S., Krynická, V., Kuppusamy, S. G., Zakar, T., Dean, J., Szilák, L., Komenda, J., Gombos, Z., and Ughy, B. (2018) Phosphatidylglycerol is implicated in divisome formation and metabolic processes of cyanobacteria, J. Plant Phys. 223:96–104.
Kanna, S. D., Domonkos, I., Kobori, T. O., Dergez, A., Bode, K., Nagyapati, S., Zsiros, O., Unnep, R., Nagy, G., Garab, G., Szilak, L., Solymosi, K., Kovacs, L., and Ughy, B. (2021) Salt Stress Induces Paramylon Accumulation and Fine-Tuning of the Macro-Organization of Thylakoid Membranes in Euglena gracilis Cells. Front. Plant. Sci. 12:725699.
We transform confocal laser scanning microscopes (LSMs) into differential-polarization (DP‑)LSMs, which, via measuring pixel-by-pixel different physical quantities allow the mapping of anisotropic molecular organization of biological samples. A DP-LSM and a Re-scan confocal microscope with differential-polarization attachment (DP-RCM) are part of the Euro-BioImaging network.
Expanding the collaboration with the ELI-ALPS laser centre (www.eli-alps.hu), we have developed a user workstation for multidimensional optical spectroscopy (MDOS) employing ultrashort, ultrabroadband pulses at very high repetition rates that will be available to the broad research community.
Time-correlated single-photon counting spectrometers (PicoQuant, Becker & Hickl)
Picosecond LED lasers (PicoQuant, Alphalas)
Picosecond supercontinuum laser (NKT Photonics)
40 ps instrument response width (FWHM)
Multidimensional optical spectroscopy workstation at ELI-ALPS:
Femtosecond transient absorption spectroscopy
2D electronic spectroscopy
UV-VIS absorption spectroscopy (double-beam spectrophotometer)
Circular and linear dichroism spectroscopy (Jasco J-815 spectropolarimeter)
Fluorescence emission and excitation spectra (Jasco FP-8500 spectrofluorometer)
Pulse-amplitude modulated fluorometer (Walz Multicolor-PAM)
Fast fluorescence induction measurements (Hansatech, PSI)
Delayed fluorescence (Hansatech M-PEA)
Thermoluminescence
Small-angle neutron scattering (www.psi.ch, www.ill.eu, mlz-garching.de)
Synchrotron-radiation circular dichroism (www.diamond.ac.uk)
Nuclear magnetic resonance spectroscopy (www.ceric-eric.eu)
THz Laboratory (https://www.eli-alps.hu/en/Users-2/NLTSF-1)
Protein purification
Reconstitution of protein complexes and membranes
FPLC (Akta) Gel electrophoresis,
Devadasu, E., Kanna, S.D., Neelam, S., Yadav, R.M., Nama, S., Akhtar, P., et al. (2023) Long- and short-term acclimation of the photosynthetic apparatus to salinity in Chlamydomonas reinhardtii. The role of Stt7 protein kinase. Front. Plant Sci. 14.
Farkas, A., Pap, B., Zsiros, O., Patai, R., Shetty, P., Garab, G., et al. (2023) Salinity stress provokes diverse physiological responses of eukaryotic unicellular microalgae. Algal Research-Biomass Biofuels and Bioproducts 73.
Garab, G., Magyar, M., Sipka, G. and Lambrev, P.H. (2023) Chlorophyll-a fluorescence induction on new grounds: quantum efficiency versus the light-adapted state of photosystem II. J. Exp. Bot.
Jajoo, A., Subramanyam, R., Garab, G. and Allakhverdiev, S.I. (2023) Honoring two stalwarts of photosynthesis research: Eva-Mari Aro and Govindjee. Photosynth. Res. 157: 43-51.
Lambreva, M.D., Akhtar, P., Sipka, G., Margonelli, A. and Lambrev, P.H. (2023) Fluorescence quenching in thylakoid membranes induced by single-walled carbon nanotubes. Photochem. Photobiol. Sci.: 1–11.
Magyar, M., Sipka, G., Han, W., Li, X., Han, G., Shen, J.-R., et al. (2023) Characterization of the Rate-Limiting Steps in the Dark-To-Light Transitions of Closed Photosystem II: Temperature Dependence and Invariance of Waiting Times during Multiple Light Reactions. International Journal of Molecular Sciences 24: 94.
Ughy, B., Nagyapati, S., Lajko, D.B., Letoha, T., Prohaszka, A., Deeb, D., et al. (2023) Reconsidering Dogmas about the Growth of Bacterial Populations. Cells 12: 1430.
Akhtar, P., Biswas, A., Balog-Vig, F., Domonkos, I., Kovács, L. and Lambrev, P.H. (2022a) Trimeric Photosystem I facilitates energy transfer from phycobilisomes in Synechocystis PCC 6803. Plant Physiol. (In press).
Akhtar, P., Sipka, G., Han, W., Li, X., Han, G., Shen, J.-R., et al. (2022b) Ultrafast excitation quenching by the oxidized Photosystem II reaction center. J. Chem. Phys. (Under revision).
Dlouhy, O., Karlicky, V., Javornik, U., Kurasova, I., Zsiros, O., Sket, P., et al. (2022a) Lipid polymorphism of plant thylakoid membranes. Structural and functional units associated with non-bilayer phases. Biochim. Biophys. Acta 1863: 83-83.
Dlouhy, O., Karlicky, V., Javornik, U., Kurasova, I., Zsiros, O., Sket, P., et al. (2022b) Structural Entities Associated with Different Lipid Phases of Plant Thylakoid Membranes-Selective Susceptibilities to Different Lipases and Proteases. Cells 11.
Do, T.N., Nguyen, H.L., Akhtar, P., Zhong, K., Jansen, T.L.C., Knoester, J., et al. (2022) Ultrafast Excitation Energy Transfer Dynamics in the LHCII–CP29–CP24 Subdomain of Plant Photosystem II. J. Phys. Chem. Lett.: 4263-4271.
Garab, G., Yaguzhinsky, L.S., Dlouhý, O., Nesterov, S.V., Špunda, V. and Gasanoff, E.S. (2022) Structural and functional roles of non-bilayer lipid phases of chloroplast thylakoid membranes and mitochondrial inner membranes. Prog. Lipid Res. 86: 101163.
Magyar, M., Akhtar, P., Sipka, G., Han, W., Li, X., Han, G., et al. (2022) Dependence of the rate-limiting steps in the dark-to-light transition of photosystem II on the lipidic environment of the reaction center. Photosynthetica 60: 147-156.
Nagy, L., Magyar, M. (2022) No alternatives to photosynthesis: From molecules to nanostructures, In Agricultural Biocatalysis: Theoretical Studies and Photosynthesis Aspects (Jeschke, P.; Starikov, E. B., Eds.) 1 ed. 211–247, Jenny Stanford Publishing.
Pleckaitis, M., Habach, F., Kontenis, L., Steinbach, G., Jarockyte, G., Kalnaityte, A., et al. (2022) Structure and principles of self-assembly of giant “sea urchin” type sulfonatophenyl porphine aggregates. Nano Research 15: 5527–5537.
Rantala, M., Ivanauskaite, A., Laihonen, L., Kanna, S.D., Ughy, B. and Mulo, P. (2022) Chloroplast Acetyltransferase GNAT2 is Involved in the Organization and Dynamics of Thylakoid Structure. Plant Cell Physiol. 63: 1205-1214.
Sipka, G., Nagy, L., Magyar, M., Akhtar, P., Shen, J.-R., Holzwarth, A.R., et al. (2022) Light-induced reversible reorganizations in closed Type II reaction centre complexes: physiological roles and physical mechanisms. Open Biology 12: 220297.
Wang, H.Y., Qin, H., Garab, G. and Gasanoff, E.S. (2022) Short-Chained Alcohols Make Membrane Surfaces Conducive for Melittin Action: Implication for the Physiological Role of Alcohols in Cells. Cells 11.
Akhtar, P., Biswas, A., Kovacs, L., Nelson, N., and Lambrev, P. H. (2021) Excitation energy transfer kinetics of trimeric, monomeric and subunit-depleted Photosystem I from Synechocystis PCC 6803. Biochemical Journal 478: 1333–1346.
Akhtar, P., Caspy, I., Nowakowski, P.J., Malavath, T., Nelson, N., Tan, H.-S. and Lambrev, P.H. (2021) Two-dimensional electronic spectroscopy of a minimal Photosystem I complex reveals the rate of primary charge separation. Journal of the American Chemical Society 143: 14601–14612.
Gasanoff, E. S., Yaguzhinsky, L. S., and Garab, G. (2021) Cardiolipin, Non-Bilayer Structures and Mitochondrial Bioenergetics: Relevance to Cardiovascular Disease. Cells-Basel 10: 1721.
Kanna, S. D., Domonkos, I., Kobori, T. O., Dergez, A., Bode, K., Nagyapati, S., Zsiros, O., Unnep, R., Nagy, G., Garab, G., Szilak, L., Solymosi, K., Kovacs, L., and Ughy, B. (2021) Salt Stress Induces Paramylon Accumulation and Fine-Tuning of the Macro-Organization of Thylakoid Membranes in Euglena gracilis Cells. Front. Plant. Sci. 12: 725699.
Karlický, V., Kmecová Materová, Z., Kurasová, I., Nezval, J., Štroch, M., Garab, G., and Špunda, V. (2021) Accumulation of geranylgeranylated chlorophylls in the pigment-protein complexes of Arabidopsis thaliana acclimated to green light: effects on the organization of light-harvesting complex II and photosystem II functions. Photosynthesis Research 149: 233–252.
Lambrev, P. H., Akhtar, P., and Garab, G. (2021) Plasticity of Photosystem II. Fine-Tuning of the Structure and Function of Light-Harvesting Complex II and the Reaction Center, In Photosynthesis: Molecular Approaches to Solar Energy Conversion (Shen, J.-R., and Satoh, K., and Allakhverdiev, S. I., Eds.) 1 ed. 375–393, Springer.
Nguyen, H. L., Do, T. N., Akhtar, P., Jansen, T. L., Knoester, J., Wang, W., Shen, J.-R., Lambrev, P. H., and Tan, H.-S. (2021) An Exciton Dynamics Model of Bryopsis corticulans Light-Harvesting Complex II. The Journal of Physical Chemistry B 125: 1134–1143.
Patty, C. H. L., Kühn, J. G., Lambrev, P. H., Spadaccia, S., Jens Hoeijmakers, H., Keller, C., Mulder, W., Pallichadath, V., Poch, O., Snik, F., Stam, D. M., Pommerol, A., and Demory, B.-O. (2021) Biosignatures of the Earth. A&A 651: A68.
Radosavljevic, J. S., Mitrovic, A. L., Radotic, K., Zimanyi, L., Garab, G., and Steinbach, G. (2021) Differential Polarization Imaging of Plant Cells. Mapping the Anisotropy of Cell Walls and Chloroplasts. Int J Mol Sci 22: 7661.
Sipka, G., Magyar, M., Mezzetti, A., Akhtar, P., Zhu, Q., Xiao, Y., Han, G., Santabarbara, S., Shen, J.-R., Lambrev, P. H., and Garab, G. (2021) Light-Adapted Charge-Separated State of Photosystem II: Structural and Functional Dynamics of the Closed Reaction Center. The Plant Cell 33: 1286–1302.
Tutkus, M., Chmeliov, J., Trinkunas, G., Akhtar, P., Lambrev, P.H. and Valkunas, L. (2021) Aggregation-related quenching of LHCII fluorescence in liposomes revealed by single-molecule spectroscopy. J. Photochem. Photobiol. B: Biol. 218: 112174.
Akhtar P, Biswas A, Petrova N, Zakar T, van Stokkum IHM, Lambrev PH (2020) Time-resolved fluorescence study of excitation energy transfer in the cyanobacterium Anabaena PCC 7120. Photosynth Res 144 (2):247–259.
Akhtar P, Nowakowski PJ, Wang W, Do TN, Zhao S, Siligardi G, Garab G, Shen JR, Tan HS, Lambrev PH (2020) Spectral tuning of light-harvesting complex II in the siphonous alga Bryopsis corticulans and its effect on energy transfer dynamics. Biochim Biophys Acta Bioenerg 1861 (7):148191.
Akhtar P, Lambrev P (2020) On the spectral properties and excitation dynamics of long-wavelength chlorophylls in higher-plant photosystem I. Biochim Biophys Acta Bioenerg 1861 (11):148274.
Bernula D, Benkő P, Kaszler N, Domonkos I, Valkai I, Szőllősi R, Ferenc G, Ayaydin F, Fehér A, Gémes K (2020) Timely removal of exogenous cytokinin and the prevention of auxin transport from the shoot to the root affect the regeneration potential of Arabidopsis roots. Plant Cell, Tissue and Organ Culture (PCTOC) 140 (2):327–339.
Biswas A, Huang X, Lambrev PH, van Stokkum IHM (2020) Modelling excitation energy transfer and trapping in the filamentous cyanobacterium Anabaena variabilis PCC 7120. Photosynth Res 144 (2):261–272.
Dlouhy O, Kurasova I, Karlicky V, Javornik U, Sket P, Petrova N Z, Krumova S B, Plavec J, Ughy B, Spunda V, Garab G (2020) Modulation of non-bilayer lipid phases and the structure and functions of thylakoid membranes: effects on the water-soluble enzyme violaxanthin de-epoxidase. Sci Rep 10 (1): 11959.
Lambrev PH, Akhtar P, Tan H-S (2020) Insights into the mechanisms and dynamics of energy transfer in plant light-harvesting complexes from two-dimensional electronic spectroscopy. Biochim Biophys Acta 1861 (4):148050.
Leng X, Do TN, Akhtar P, Nguyen HL, Lambrev P, Tan HS (2020) Hierarchical Equations of Motion Simulation of Temperature-Dependent Two-Dimensional Electronic Spectroscopy of the Chlorophyll a Manifold in LHCII. Chem Asian J in press.
Lingvay M, Akhtar P, Sebok-Nagy K, Pali T, Lambrev P (2020) Photobleaching of Chlorophyll in Light-Harvesting Complex II Increases in Lipid Environment. Front Plant Sci 849 (11):14 p.
Nagy, G., and Garab, G. (2020) Neutron scattering in photosynthesis research: recent advances and perspectives for testing crop plants, Photosynthesis Research 150: 41–49.
Ostroumov EE, Gotze JP, Reus M, Lambrev PH, Holzwarth AR (2020) Characterization of fluorescent chlorophyll charge-transfer states as intermediates in the excited state quenching of light-harvesting complex II. Photosynth Res 144 (2):171–193.
Ünnep R, Paul S, Zsiros O,Kovács L, Székely NK, Steinbach G, Appavou M-S, Porcar L, Holzwarth AR, Garab G, Nagy G (2020) Thylakoid membrane reorganizations revealed by small-angle neutron scattering of Monstera deliciosa leaves associated with non-photochemical quenching. Open biol 200144 , 12 p.
Wientjes E, Lambrev P (2020) Ultrafast processes in photosynthetic light-harvesting. Photosynth Res 144 (2):123–125.
Wilhelm, C., Goss, R., and Garab, G. (2020) The fluid-mosaic membrane theory in the context of photosynthetic membranes: Is the thylakoid membrane more like a mixed crystal or like a fluid?. J Plant Physiol 252: 153246.
Zsiros O, Ünnep R, Nagy G, Almasy L, Patai R, Szekely NK, Kohlbrecher J, Garab G, Der A, Kovacs L (2020) Role of Protein-Water Interface in the Stacking Interactions of Granum Thylakoid Membranes-As Revealed by the Effectsof Hofmeister Salts. Front Plant Sci 1257 (11):14 p.
Zsiros O, Nagy G, Patai R, Solymosi K, Gasser U, Polgar TF, Garab G, Kovacs L, Horcsik ZT (2020) Similarities and Differences in the Effects of Toxic Concentrations of Cadmium and Chromium on the Structure and Functions of Thylakoid Membranes in Chlorella variabilis. Front Plant Sci 1006, 13 p.
Akhtar P, Do TN, Nowakowski PJ, Huerta-Viga A, Khyasudeen MF, Lambrev PH, Tan HS (2019) Temperature Dependence of the Energy Transfer in LHCII Studied by Two-Dimensional Electronic Spectroscopy. J Phys Chem B 123 (31):6765–6775.
Akhtar P, Gorfol F, Garab G, Lambrev PH (2019) Dependence of chlorophyll fluorescence quenching on the lipid-to-protein ratio in reconstituted light-harvesting complex II membranes containing lipid labels. Chem Phys 522:242–248.
Akhtar P, Lindorfer D, Lingvay M, Pawlak K, Zsiros O, Siligardi G, Javorfi T, Dorogi M, Ughy B, Garab G, Renger T, Lambrev PH (2019) Anisotropic Circular Dichroism of Light-Harvesting Complex II in Oriented Lipid Bilayers: Theory Meets Experiment. J Phys Chem B 123 (5):1090–1098.
Bocsik A, Grof I, Kiss L, Otvos F, Zsiros O, Daruka L, Fulop L, Vastag M, Kittel A, Imre N, Martinek TA, Pal C, Szabo-Revesz P, Deli MA (2019) Dual Action of the PN159/KLAL/MAP Peptide: Increase of Drug Penetration across Caco-2 Intestinal Barrier Model by Modulation of Tight Junctions and Plasma Membrane Permeability. Pharmaceutics 11 (2):73.
Do TN, Huerta-Viga A, Akhtar P, Nguyen HL, Nowakowski PJ, Khyasudeen MF, Lambrev PH, Tan H-S (2019) Revealing the excitation energy transfer network of Light-Harvesting Complex II by a phenomenological analysis of two-dimensional electronic spectra at 77 K. J Chem Phys 151 (20):205101.
Hudak A, Kusz E, Domonkos I, Josvay K, Kodamullil AT, Szilak L, Hofmann-Apitius M, Letoha T (2019) Contribution of syndecans to cellular uptake and fibrillation of alpha-synuclein and tau. Scientific Reports 9:16543.
Lambrev PH, Akhtar P (2019) Macroorganisation and flexibility of thylakoid membranes. Biochem J 476 (20):2981–3018.
Letoha T, Hudak A, Kusz E, Pettko-Szandtner A, Domonkos I, Josvay K, Hofmann-Apitius M, Szilak L (2019) Contribution of syndecans to cellular internalization and fibrillation of amyloid-beta(1-42). Scientific Reports 9 (1):1393.
Patty CHL, ten Kate IL, Buma WJ, van Spanning RJM, Steinbach G, Ariese F, Snik F (2019) Circular Spectropolarimetric Sensing of Vegetation in the Field: Possibilities for the Remote Detection of Extraterrestrial Life. Astrobiology 19 (10):1221–1229.
Sipka G, Muller P, Brettel K, Magyar M, Kovacs L, Zhu QJ, Xiao YA, Han GY, Lambrev PH, Shen JR, Garab G (2019) Redox transients of P680 associated with the incremental chlorophyll-a fluorescence yield rises elicited by a series of saturating flashes in diuron-treated photosystem II core complex of Thermosynechococcus vulcanus. Physiol Plant 166 (1):22–32.
Steinbach G, Nagy D, Sipka G, Manders E, Garab G, Zimanyi L (2019) Fluorescence-detected linear dichroism imaging in a re-scan confocal microscope equipped with differential polarization attachment. Eur Biophys J 48 (5):457–463.
Ughy B, Karlicky V, Dlouhy O, Javornik U, Materova Z, Zsiros O, Sket P, Plavec J, Spunda V, Garab G (2019) Lipid-polymorphism of plant thylakoid membranes. Enhanced non-bilayer lipid phases associated with increased membrane permeability. Physiol Plant 166 (1):278–287.
Ughy B, Schmidthoffer I, Szilak L (2019) Heparan sulfate proteoglycan (HSPG) can take part in cell division: inside and outside. Cell Mol Life Sci 76 (5):865–871.
Zsiros O, Nagy V, Párducz Á, Nagy G, Ünnep R, El-Ramady H, Prokisch J, Lisztes-Szabó Z, Fári M, Csajbók J, Tóth SZ, Garab G, Domokos-Szabolcsy É (2019) Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth Res 139 (1):449-460.
Akhtar P, Zhang C, Liu Z, Tan HS, Lambrev PH (2018) Excitation transfer and trapping kinetics in plant photosystem I probed by two-dimensional electronic spectroscopy. Photosynth Res 135 (1–3):239–250.
Domokos-Szabolcsy É, Fári M, Márton L, Czakó M, Veres S, Elhawat N, Antal G, El-Ramady H, Zsíros O, Garab G (2018) Selenate tolerance and selenium hyperaccumulation in the monocot giant reed (Arundo donax), a biomass crop plant with phytoremediation potential. Environ Sci Pollut Res 25 (31):31368–31380.
Erdei AI, Borbely A, Magyar A, Taricska N, Perczel A, Zsiros O, Garab G, Szucs E, Otvos F, Zador F, Balogh M, Al-Khrasani M, Benyhe S (2018) Biochemical and pharmacological characterization of three opioid-nociceptin hybrid peptide ligands reveals substantially differing modes of their actions. Peptides 99:205–216.
Kis M, Sipka G, Ayaydin F, Maroti P (2018) The biophysics of a critical phenomenon: colonization and sedimentation of the photosynthetic bacteria Rubrivivax gelatinosus. Eur Biophys J 47 (2):139–149.
Kóbori TO, Uzumaki T, Kis M, Kovács L, Domonkos I, Itoh S, Krynická V, Kuppusamy SG, Zakar T, Dean J, Szilák L, Komenda J, Gombos Z, Ughy B (2018) Phosphatidylglycerol is implicated in divisome formation and metabolic processes of cyanobacteria. J Plant Physiol 223:96–104.
Kotakis C, Akhtar P, Zsiros O, Garab G, Lambrev PH (2018) Increased thermal stability of photosystem II and the macro-organization of thylakoid membranes, induced by co-solutes, associated with changes in the lipid-phase behaviour of thylakoid membranes. Photosynthetica.
Magyar M, Sipka G, Kovács L, Ughy B, Zhu Q, Han G, Špunda V, Lambrev PH, Shen J-R, Garab G (2018) Rate-limiting steps in the dark-to-light transition of Photosystem II - revealed by chlorophyll-a fluorescence induction. Scientific Reports 8 (1):2755.
Nedzved A, Mitrović AL, Savić A, Mutavdžić D, Radosavljević JS, Pristov JB, Steinbach G, Garab G, Starovoytov V, Radotić K (2018) Automatic image processing morphometric method for the analysis of tracheid double wall thickness tested on juvenile Picea omorika trees exposed to static bending. Trees 32 (5):1347–1356.
Patty CL, Luo DA, Snik F, Ariese F, Buma WJ, Ten Kate IL, van Spanning RJ, Sparks WB, Germer TA, Garab G (2018) Imaging linear and circular polarization features in leaves with complete Mueller matrix polarimetry. Biochimica et Biophysica Acta (BBA) - General Subjects 1862 (6):1350–1363.
Sipka G, Kis M, Maroti P (2018) Characterization of mercury(II)-induced inhibition of photochemistry in the reaction center of photosynthetic bacteria. Photosynth Res. 136: 379–392.
Sipka G, Kis M, Smart JL, Maróti P (2018) Fluorescence induction of photosynthetic bacteria. Photosynthetica 56 (1):125–131.
Sipka G, Maroti P (2018) Photoprotection in intact cells of photosynthetic bacteria: quenching of bacteriochlorophyll fluorescence by carotenoid triplets. Photosynth Res 136 (1):17–30.
Stefanov D, Milanov G, Lambrev P, Kurteva M, Abumhadi N, Goltsev V, Kapchina V (2018) Delayed fluorescence measurements show increased S2QB− charge recombination in PS2 of tobacco pigment-deficient aurea mutant. Comptes Rendus de L'Academie Bulgare des Sciences 71 (8):1052–1061.
Tutkus M, Akhtar P, Chmeliov J, Görföl F, Trinkunas G, Lambrev PH, Valkunas L (2018) Fluorescence Microscopy of Single Liposomes with Incorporated Pigment–Proteins. Langmuir 34 (47):14410–14418.
Akhtar P, Zhang C, Do TN, Garab G, Lambrev PH, Tan H-S (2017) Two-dimensional spectroscopy of chlorophyll a excited-state equilibration in light-harvesting complex II. J Phys Chem Lett 8 (1):257–263.
Bar Eyal L, Ranjbar Choubeh R, Cohen E, Eisenberg I, Tamburu C, Dorogi M, Unnep R, Appavou MS, Nevo R, Raviv U, Reich Z, Garab G, van Amerongen H, Paltiel Y, Keren N (2017) Changes in aggregation states of light-harvesting complexes as a mechanism for modulating energy transfer in desert crust cyanobacteria. Proceedings of the National Academy of Sciences 114 (35):9481–9486.
Garab G, Ughy B, de Waard P, Akhtar P, Javornik U, Kotakis C, Sket P, Karlicky V, Materova Z, Spunda V, Plavec J, van Amerongen H, Vigh L, Van As H, Lambrev PH (2017) Lipid polymorphism in chloroplast thylakoid membranes - as revealed by 31P-NMR and timeresolved merocyanine fluorescence spectroscopy. Scientific Reports 7 (1):13343.
Keller-Pinter A, Ughy B, Domoki M, Pettko-Szandtner A, Letoha T, Tovari J, Timar J, Szilak L (2017) The phosphomimetic mutation of syndecan-4 binds and inhibits Tiam1 modulating Rac1 activity in PDZ interaction-dependent manner. PLoS One 12 (11):e0187094.
Radosavljevic JS, Pristov JB, Mitrovic AL, Steinbach G, Mouille G, Tufegdzic S, Maksimovic V, Mutavdzic D, Janosevic D, Vukovic M, Garab G, Radotic K (2017) Parenchyma cell wall structure in twining stem of Dioscorea balcanica. Cellulose 24 (11):4653–4669.
Szabo T, Cseko R, Hajdu K, Nagy K, Sipos O, Galajda P, Garab G, Nagy L (2017) Sensing photosynthetic herbicides in an electrochemical flow cell. Photosynth Res 132 (2):127–134.
Unnep R, Zsiros O, Horcsik Z, Marko M, Jajoo A, Kohlbrecher J, Garab G, Nagy G (2017) Low-pH induced reversible reorganizations of chloroplast thylakoid membranes - As revealed by small-angle neutron scattering. Bba-Bioenergetics 1858 (5):360–365.
Akhtar P, Lingvay M, Kiss T, Deák R, Bóta A, Ughy B, Garab G, Lambrev PH (2016) Excitation energy transfer between light-harvesting complex II and photosystem I in reconstituted membranes. Biochim Biophys Acta 1857 (4):462–472.
Garab G (2016) Self-assembly and structural–functional flexibility of oxygenic photosynthetic machineries: personal perspectives. Photosynth Res 127 (1):131-150.
Garab G, Ughy B, Goss R (2016) Role of MGDG and Non-bilayer Lipid Phases in the Structure and Dynamics of Chloroplast Thylakoid Membranes. In: Nakamura Y, Li-Beisson Y (eds) Lipids in Plant and Algae Development. Springer, Dordrecht, pp 127–157. doi:10.1007/978-3-319-25979-6
Ghazaryan A, Akhtar P, Garab G, Lambrev PH, Buchel C (2016) Involvement of the Lhcx protein Fcp6 of the diatom Cyclotella meneghiniana in the macro-organisation and structural flexibility of thylakoid membranes. Biochim Biophys Acta 1857 (9):1373–1379.
Herdean A, Nziengui H, Zsiros O, Solymosi K, Garab G, Lundin B, Spetea C (2016a) The Arabidopsis Thylakoid Chloride Channel AtCLCe Functions in Chloride Homeostasis and Regulation of Photosynthetic Electron Transport. Front Plant Sci 7:115.
Herdean A, Teardo E, Nilsson AK, Pfeil BE, Johansson ON, Unnep R, Nagy G, Zsiros O, Dana S, Solymosi K, Garab G, Szabo I, Spetea C, Lundin B (2016b) A voltage-dependent chloride channel fine-tunes photosynthesis in plants. Nat Commun 7:11654.
Nielsen JT, Kulminskaya NV, Bjerring M, Linnanto JM, Ratsep M, Pedersen MO, Lambrev PH, Dorogi M, Garab G, Thomsen K, Jegerschold C, Frigaard NU, Lindahl M, Nielsen NC (2016) In situ high-resolution structure of the baseplate antenna complex in Chlorobaculum tepidum. Nat Commun 7:12454.
Tóth TN, Rai N, Solymosi K, Zsiros O, Schröder WP, Garab G, van Amerongen H, Horton P, Kovács L (2016) Fingerprinting the macro-organisation of pigment-protein complexes in plant thylakoid membranes in vivo by circular-dichroism spectroscopy. Biochimica et Biophysica Acta-Bioenergetics 1857 (9):1479–1489.
Akhtar P, Dorogi M, Pawlak K, Kovacs L, Bota A, Kiss T, Garab G, Lambrev PH (2015) Pigment interactions in light-harvesting complex II in different molecular environments. J Biol Chem 290 (8):4877–4886.
Bricker WP, Shenai PM, Ghosh A, Liu Z, Enriquez MG, Lambrev PH, Tan HS, Lo CS, Tretiak S, Fernandez-Alberti S, Zhao Y (2015) Non-radiative relaxation of photoexcited chlorophylls: theoretical and experimental study. Sci Rep 5:13625.
Enriquez MM, Akhtar P, Zhang C, Garab G, Lambrev PH, Tan HS (2015) Energy transfer dynamics in trimers and aggregates of light-harvesting complex II probed by 2D electronic spectroscopy. J Chem Phys 142 (21):212432.
Karlsson PM, Herdean A, Adolfsson L, Beebo A, Nziengui H, Irigoyen S, Unnep R, Zsiros O, Nagy G, Garab G, Aronsson H, Versaw WK, Spetea C (2015) The Arabidopsis thylakoid transporter PHT4;1 influences phosphate availability for ATP synthesis and plant growth. Plant J 84 (1):99–110.
Nagy L, Kiss V, Brumfeld V, Osvay K, Borzsonyi A, Magyar M, Szabo T, Dorogi M, Malkin S (2015) Thermal Effects and Structural Changes of Photosynthetic Reaction Centers Characterized by Wide Frequency Band Hydrophone: Effects of Carotenoids and Terbutryn. Photochem Photobiol 91 (6):1368–1375.
Szabo T, Magyar M, Hajdu K, Dorogi M, Nyerki E, Toth T, Lingvay M, Garab G, Hernadi K, Nagy L (2015) Structural and Functional Hierarchy in Photosynthetic Energy Conversion-from Molecules to Nanostructures. Nanoscale Res Lett 10 (1):458.
Toth TN, Chukhutsina V, Domonkos I, Knoppova J, Komenda J, Kis M, Lenart Z, Garab G, Kovacs L, Gombos Z, van Amerongen H (2015) Carotenoids are essential for the assembly of cyanobacterial photosynthetic complexes. Biochim Biophys Acta 1847 (10):1153–1165.
Zhang Z, Lambrev PH, Wells KL, Garab G, Tan HS (2015) Direct observation of multistep energy transfer in LHCII with fifth-order 3D electronic spectroscopy. Nat Commun 6:7914.
Demmig-Adams, B., Garab, G. & Adams III, W. (Eds) (2014) Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Adv. Photosynth. Respir., Vol. 40, Springer Science+Business Media, Dordrecht
Garab, G. (2014) Hierarchical organization and structural flexibility of thylakoid membranes. Biochim. Biophys. Acta 1837: 481-494.
Hind, G., Wall, J.S., Várkonyi, Z., Istokovics, A., Lambrev, P.H. & Garab, G. (2014) Membrane crystals of plant light-harvesting complex II disassemble reversibly in light. Plant Cell Physiol. 55: 1296-303.
Nagy, G., Ünnep, R., Zsiros, O., Tokutsu, R., Takizawa, K., Porcar, L., Moyet, L., Petroutsos, D., Garab, G., Finazzi, G. & Minagawa, J. (2014) Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by non-invasive techniques in vivo, Proc. Natl. Acad. Sci. USA, 111: 5042-5047.
Ünnep, R., Zsiros, O., Solymosi, K., Kovács, L., Lambrev, P.H., Tóth, T., Schweins, R., Posselt, D., Székely, N.K., Rosta, L., Nagy, G. & Garab G. (2014) The ultrastructure and flexibility of thylakoid membranes in leaves and isolated chloroplasts as revealed by small-angle neutron scattering. Biochim. Biophys. Acta 1837: 1572-1580.
Wells, K.L., Lambrev, P.H., Zhang, Z., Garab, G. & Tan, H.S. (2014) Pathways of energy transfer in LHCII revealed by room-temperature 2D electronic spectroscopy, Phys. Chem. Chem. Phys. 16: 1463-9076.
Lambrev, P.H., Miloslavina, Y., Van Stokkum, I.H.M., Stahl, A.D., Michalik, M., Susz, A., Tworzydło, J., Fiedor, J., Huhn, G., Groot, M.L., Van Grondelle, R., Garab, G. & Fiedor, L. (2013) Excitation energy trapping and dissipation by Ni-substituted bacteriochlorophyll a in reconstituted LH1 complexes from Rhodospirillum rubrum. J. Phys. Chem. B 117: 11260-11271.
Lambrev, P.H. & Miloslavina, Y. (2012) On the relationship between non-photochemical quenching and photoprotection of Photosystem II. Biochim. Biophys. Acta 1817: 760–769.
Nagy, G., Szabó, M., Ünnep, R., Káli, G., Miloslavina, Y., Lambrev, P.H., Zsiros, O., Porcar, L., Timmins, P., Rosta, L. & Garab, G. (2012) Modulation of the multilamellar membrane organization and of the chiral macrodomains in the diatom revealed by small-angle neutron scattering and circular dichroism spectroscopy. Photosynth. Res. 111: 71-79.
Lambrev, P.H., Schmitt, F.J., Kussin, S., Schoengen, M., Várkonyi, Zs., Eichler, H.J., Garab, G. & Renger G. (2011) Functional domain size in aggregates of light-harvesting complex II and thylakoid membranes. Biochim. Biophys. Acta 1807: 1022-1031.
Miloslavina, Y., Lambrev, P.H., Jávorfi, T., Várkonyi, Zs., Karlický, V., Wall, J.S., Hind, G. & Garab, G. (2011) Anisotropic circular dichroism signatures of oriented thylakoid membranes and lamellar aggregates of LHCII. Photosynth. Res. 111: 29-39.
Schansker, G., Tóth, S.Z., Kovács, L., Holzwarth, A.R. & Garab, G. (2011) Evidence for a fluorescence yield change driven by a light-induced conformational change within photosystem II during the fast chlorophyll a fluorescence rise. Biochim. Biophys. Acta 1807: 1032-1043.
Krumova, S.B., Laptenok, S.P., Kovács, L., Tóth, T., van Hoek, A., Garab, G. & van Amerongen, H. (2010) Digalactosyl-diacylglycerol-deficiency lowers the thermal stability of thylakoid membranes. Photosynth. Res. 105: 229-242.
Miloslavina, Y., Grouneva, I., Lambrev, P.H., Lepetit, B., Goss, R., Wilhelm, C. & Holzwarth, A.R. (2009) Ultrafast fluorescence study on the location and mechanism of non-photochemical quenching in diatoms. Biochim. Biophys. Acta 1787: 1189-1197.
Krumova, S.B., Koehorst, R.B.M., Bóta, A., Páli, T., van Hoek, A., Garab, G. & van Amerongen, H. (2008) Temperature dependence of the lipid packing in thylakoid membranes studied by time-and spectrally resolved fluorescence of Merocyanine 540. Biochim. Biophys. Acta 1778: 2823-2833.
Miloslavina, Y., Wehner, A., Lambrev, P.H., Wientjes, E., Reus, M., Garab, G., Croce, R. & Holzwarth, A.R. (2008) Far-red fluorescence: a direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett. 582: 3625-3631.
Mustárdy, L., Buttle, K., Steinbach, G. & Garab, G. (2008) The three-dimensional network of the thylakoid membranes in plants: quasihelical model of the granum-stroma assembly. Plant Cell 20: 2552-2557.
Lambrev, P.H., Várkonyi, Zs., Krumova, S., Kovács, L., Miloslavina, Y., Holzwarth, A.R. & Garab, G. (2007) Importance of trimer–trimer interactions for the native state of the plant light-harvesting complex II. Biochim. Biophys. Acta 1767: 847-853.
Gulbinas, V., Karpicz, R., Garab, G. & Valkunas, L. (2006) Nonequilibrium heating in LHCII complexes monitored by ultrafast absorbance transients. Biochemistry 45: 9559-9565.
Mustárdy, L. & Garab, G. (2003) Granum revisited. A three-dimensional model–where things fall into place. Trends Plant Sci. 8: 117-122.
Garab, G. & Mustárdy, L. (2000) Role of LHCII-containing macrodomains in the structure, function and dynamics of grana. Funct. Plant Biol. 27: 279-279.

scientific advisor

emeritus scientific adviser

senior research associate

research associate

research associate

research associate

research assistant

junior research associate

Ph.D. student

Ph.D. student

guest researcher

guest researcher
 Petar LAMBREV
Petar LAMBREV
|
scientific advisor | publications | CV |
 Győző GARAB
Győző GARAB
|
emeritus scientific adviser | publications | CV |
 Bettina UGHY
Bettina UGHY
|
senior research associate | publications | CV |
 Parveen AKHTAR
Parveen AKHTAR
|
research associate | publications | CV |
 Melinda MAGYAR
Melinda MAGYAR
|
research associate | publications | CV |
 Ildikó RACSKÓNÉ DOMONKOS
Ildikó RACSKÓNÉ DOMONKOS
|
research associate | publications | CV |
 Ottó ZSIROS
Ottó ZSIROS
|
research assistant | publications | CV |
 Kinga BÖDE
Kinga BÖDE
|
junior research associate | publications | CV |
 Dima DEEB
Dima DEEB
|
Ph.D. student | publications | CV |
 Sarolta NAGYAPÁTI
Sarolta NAGYAPÁTI
|
Ph.D. student | publications | CV |
 László NAGY
László NAGY
|
guest researcher | publications | CV |
 László SZILÁK
László SZILÁK
|
guest researcher | publications | CV |