
The immune response in Drosophila
The fundamental elements of the innate immune system originated early in animal evolution before the division of Metazoa to Protostomes and Deuterostomes, because several components of the insect innate immune system show structural and functional homology to mammalian immune cells and molecules. Given these homologies, the exploration of the Drosophila immune system has the translational potential to identify novel candidate genes for disease resistance or susceptibility, therefore any information is of direct relevance to medical science and for development of novel therapeutic targets and therapies.
Applying our experimental system we explore new areas of the innate immune response:
1.) Recently, in several Drosophila species we identified a novel blood cell type, the multinucleated giant hemocyte (MGH) (doi: 10.1159/000369618) (Figure 1), which is involved in a highly efficient elimination of parasitoids. Interestingly, the immune response of these species is fundamentally different from the well explored canonic form of anti-parasitoid defense used by Drosophila melanogaster (Figure 2). These species lack the canonical encapsulating cell type of the melanogaster subgroup, the lamellocyte, and lack prophenoloxidase-3, the key enzyme responsible for melanotic capsule formation and parasitoid elimination, suggesting that in several species outside the melanogaster subgroup a highly efficient, ancient killing mechanism with so far unidentified components is operating. A deeper analysis of Drosophila ananassae genome assembly revealed that two toxin genes, encoding the cytolethal distending toxin B (CdtB) and the apoptosis-inducing protein of 56 kDa (AIP56) were horizontally transferred from prokaryotes to insects, where their expression is induced upon infection (doi: 10.1073/pnas.2218334120). By knocking out these genes we found that they are important modules required for an efficient defense against parasitoid wasps (Figure 3). Furthermore, single cell transcriptome of D. ananassae hemocytes revealed that several genes, belonging to an orphan gene family, which encode for members of Hemolysin E family are highly expressed in the MGHs (doi: 10.1159/000520110). Hemolysin E proteins are pore forming toxins, virulence factors produced by bacteria, causing lysis of eukaryotic cells through transmembrane pore-formation. As the cdtB, aip56 and hemolysisn E genes of D. ananassae have no orthologs in D. melanogaster, we concluded that they could be involved in the high resistance to parasitoid infection of these species. There are still a few major question to be answered: how these toxins, which in bacteria served as virulence factors to kill eukaryotic cells, turned to defense factors against parasites and how D. ananassae avoids autotoxicity while deploying the toxic proteins. We further analyze how these genes were integrated into the eukaryotic genome, how they are regulated and how cooperate with the other elements of the immune system. These mechanisms are going to be further analyzed by biochemical and genetic techniques.
2.) We use integrative approaches that combine genetic and functional data with immunological phenotypes to further characterize the immune compartments and defense reactions of the invasive Drosophila species Zaprionus indianus (doi: 10.1159/000502646).
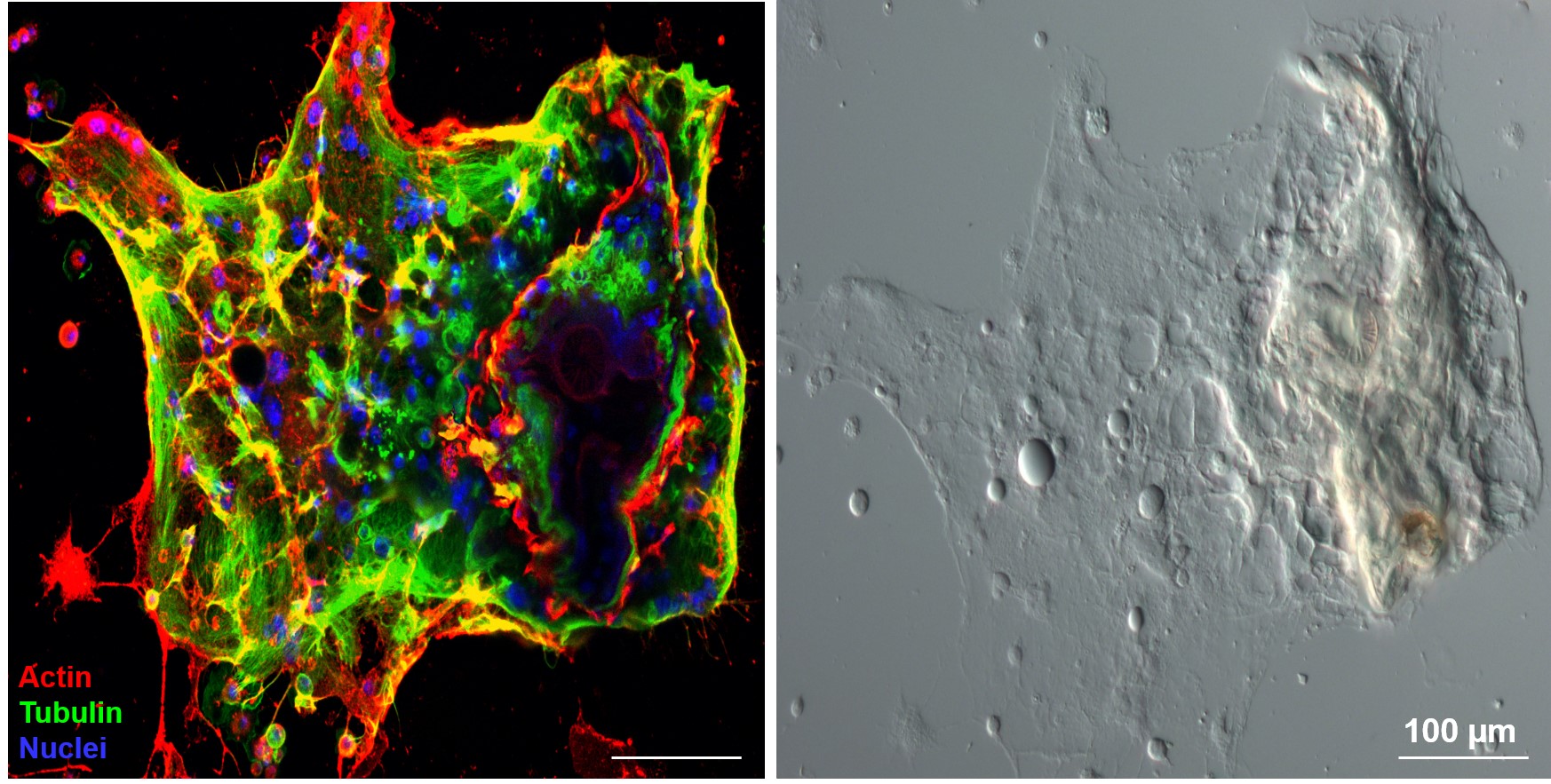
Figure 1. Multinucleated giant hemocytes encapsulating a parasitoid
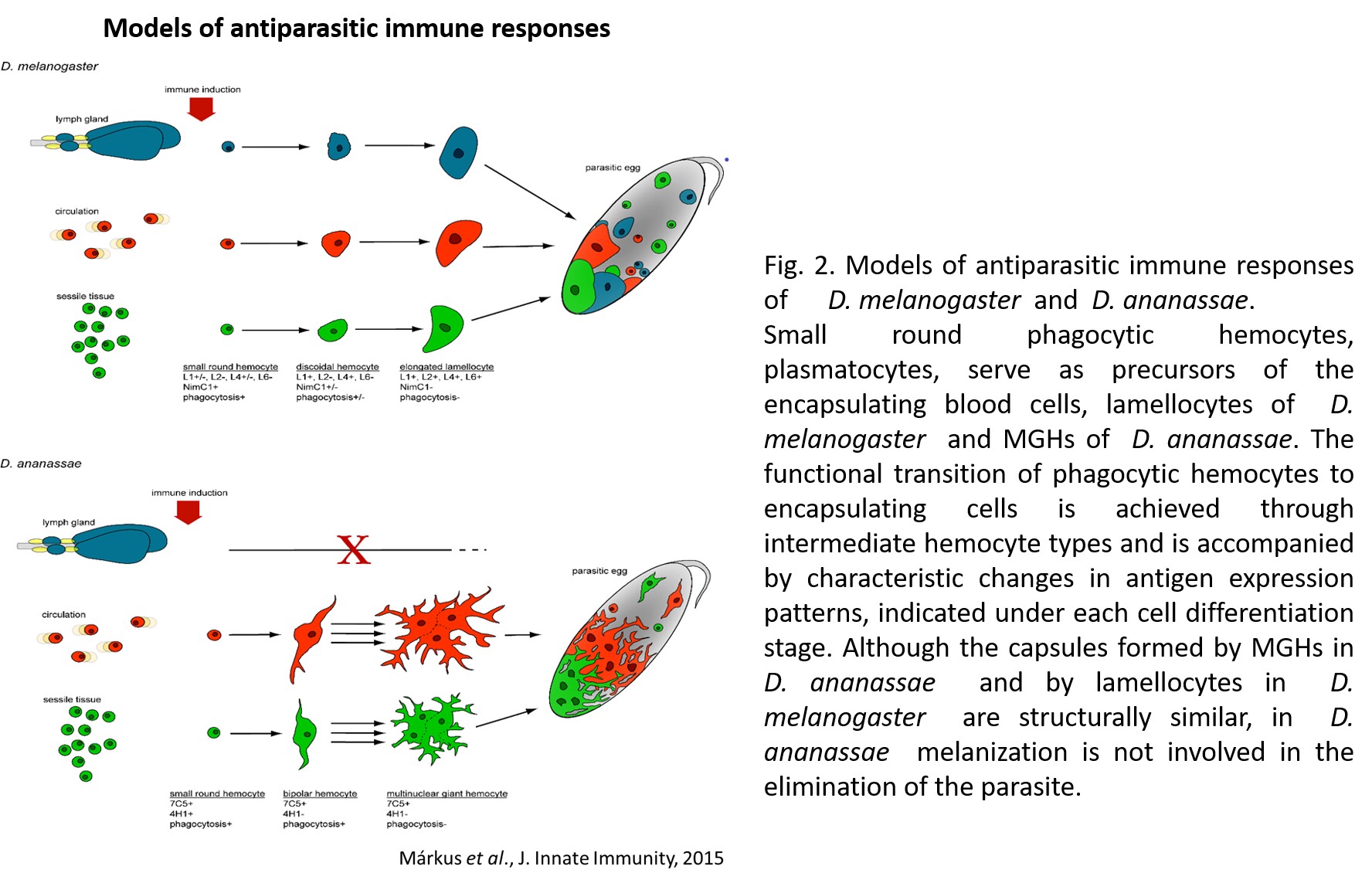
Figure 2. Antiparasitic immune responses of D. melanogaster and D. ananassae
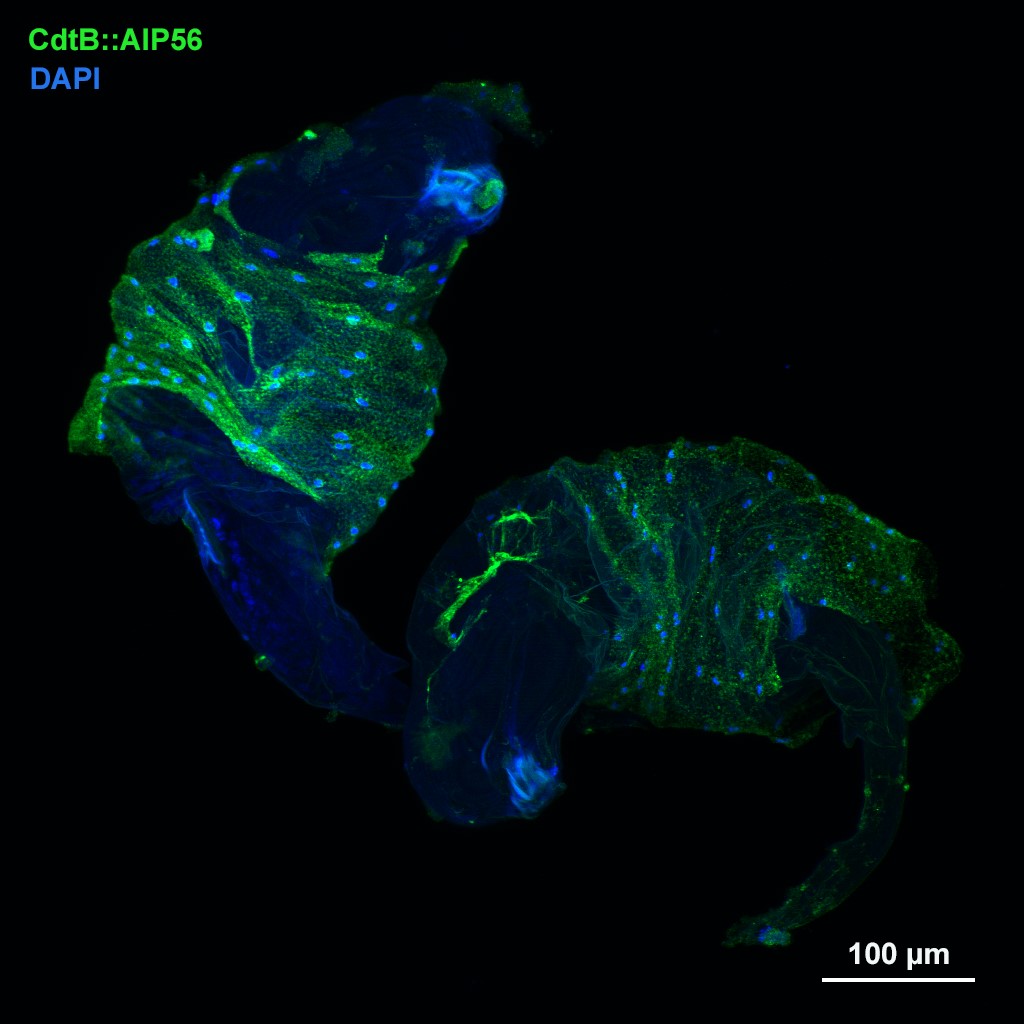
Figure 3. The CdtB::AIP56 toxin proteins attached to the parasitoid wasps
Publications:
Márkus R, Lerner Z, Honti V, Csordás G, Zsámboki J, Cinege G, Párducz Lukacsovich T, Kurucz E, Andó Multinucleated giant hemocytes are effector cells in cell-mediated immune responses of Drosophila. J Innate Immun (2015) 7, 340-53. doi: 10.1159/000369618
Cinege G, Lerner Z, Magyar LB, Soós B, Tóth R, Kristó I, Vilmos P, Juhász G, Kovács AL, Hegedűs Z, Sensen CW, Kurucz É, Andó I. Cellular immune response involving multinucleated giant hemocytes with two-step genome amplification in the drosophilid Zaprionus indianus. J Innate Immun (2020) 25, 1-16. doi: 10.1159/000502646
Cinege G, Magyar LB, Kovács AL, Lerner Z, Juhász G, Lukacsovich D, Winterer J, Lukacsovich T, Hegedűs Z, Kurucz É, Hultmark D, Földy C, Andó I. Broad ultrastructural and transcriptomic changes underlie the multinucleated giant hemocyte mediated innate immune response against parasitoids. J Innate Immun (2022) 14(4), 335-354. doi: 10.1159/000520110
Hultmark D, Andó I. Hematopoietic plasticity mapped in Drosophila and other insects. Elife (2022) 3;11:e78906. doi: 10.7554/eLife.78906
Verster, Cinege, , Magyar, Kurucz, Tarnopol, Ábrahám, Darula, , , Akalu, Andó, Whiteman. Evolution of insect innate immunity through domestication of bacterial toxins. PNAS (2023) 120(16), e2218334120.

professor emeritus

scientific adviser
 István, ANDÓ
István, ANDÓ
|
professor emeritus | publications | CV |
 Judit Éva, KURUCZ
Judit Éva, KURUCZ
|
scientific adviser | publications | CV |