
The immune system is the main defense barrier that protects organisms against parasites, pathogens and harmful effects. The fruit fly Drosophila melanogaster has been used to study immunity and blood cell differentiation for the past 60 years. Although, according to our recent knowledge, Drosophila lacks adaptive immunity, the functions of its immune cells (the hemocytes), such as phagocytosis, encapsulation and coagulation are similar to those of vertebrate myeloid cells. The signaling pathways, as well as the transcription and epigenetic factors that regulate blood cell differentiation are highly conserved. Similarly to vertebrate blood cells, hemocytes differentiate in spatially separated hematopoietic compartments in multiple waves. Therefore, Drosophila is an ideal model organism to study the regulation of stem cell function in the hematopoietic niche, as well as tumor formation caused by the misregulation of blood cell fate.
1. Transdifferentiation of Drosophila blood cells
Transdifferentiation is the conversion of an already differentiated cell type into another cell type without the involvement of stem cells. Our transgenic cell lineage tracing experiments revealed that the phagocytic plasmatocytes of the larva are plastic cells that are capable of differentiating into capsule-forming lamellocytes upon immune induction. During this conversion, plasmatocytes undergo dramatic morphological alterations and gene expression changes. To sudy transdifferentiation, we set up an ex vivo hemocyte culturing and differentiation method, which enables the time-lapse microscopy of blood cells and the detailed, high-throughput analysis of morphological changes with machine learning. Our aim is to correlate hemocyte morphology with transcriptomic changes to better understand the transdifferentiation process.
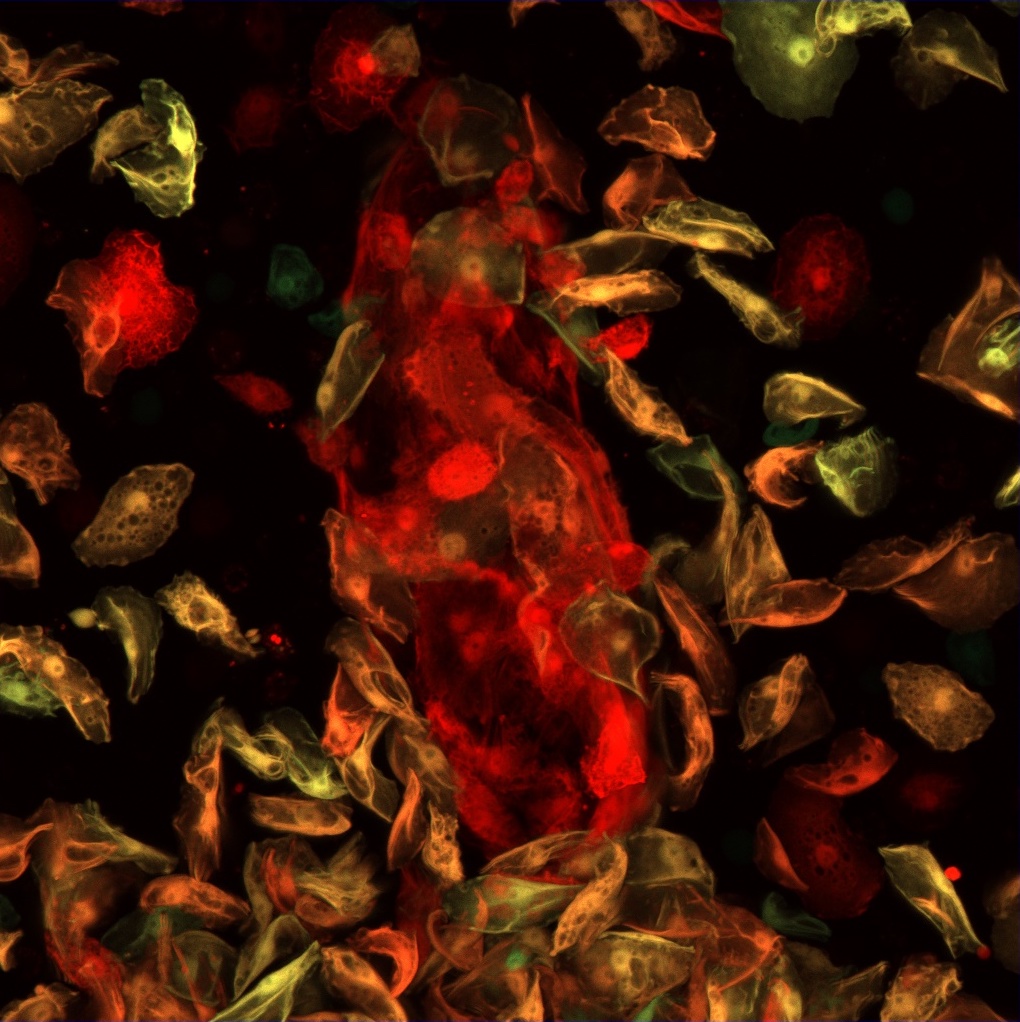
2. Regulation of blood cell progenitor maintenance
In our experiments, we have identified factors that play a significant role in the regulation of blood cell progenitor maintenance and differentiation. One of these factors is Headcase, the Drosophila homolog of the human tumor suppressor HECA, which regulates hemocyte fate in the lymph gland via modulation of Hedgehog, Decapentaplegic and JAK/STAT signaling. Our further studies aim to identify the functional domains and interacting partners of Headcase. Our goal is to shed light on the details of the complex regulatory network that ensures blood cell differentiation as well as to reveal functional similarities of Drosophila and mammalian blood cell niches.
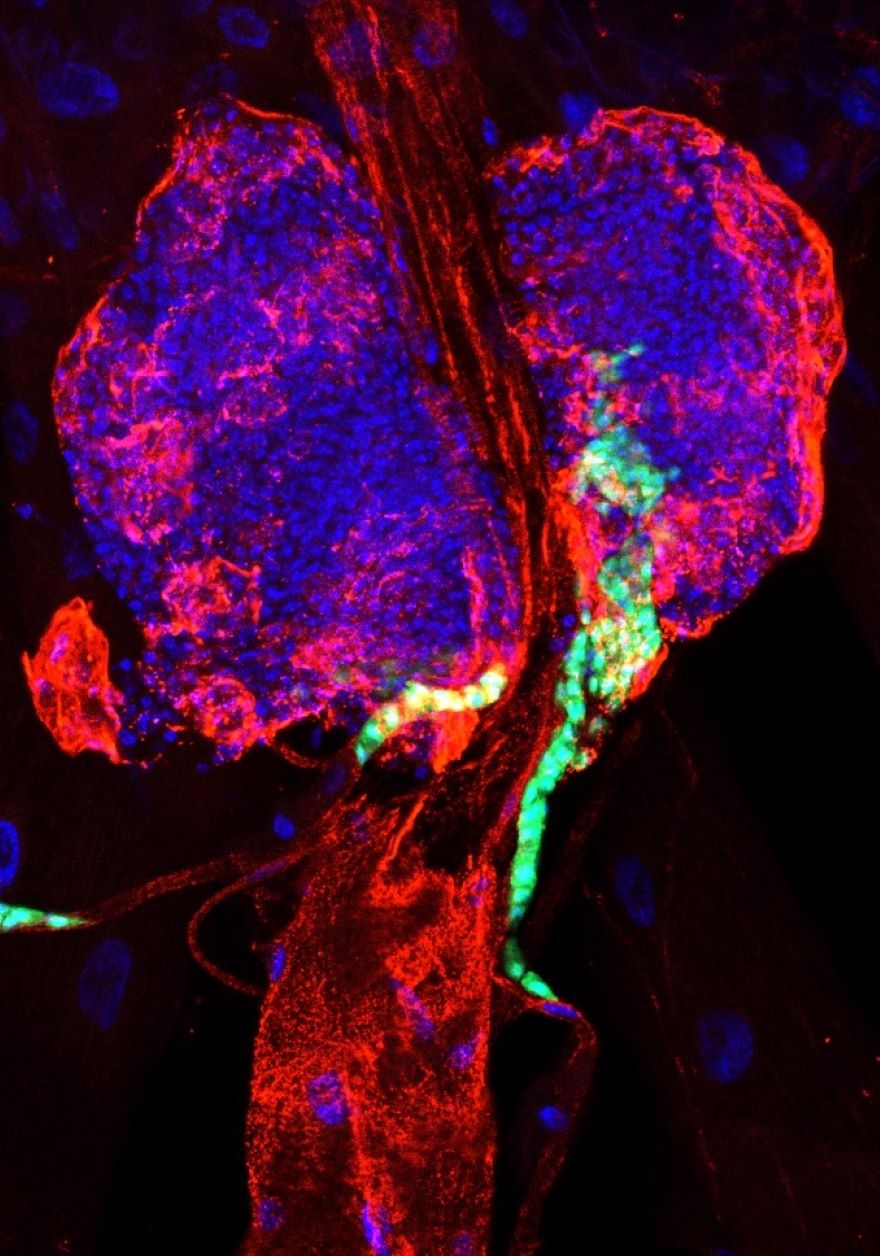
3. Novel therapeutic agents in leukemia
Leukemia can affect individuals of all age groups, with approximately half of all cancer cases in young people being attributed to this disease. The cause of leukemia is the overproliferation and tumorous transformation of blood cell progenitors. To develop more effective ways of treatment, it is crucial to gain insight into the genetic background and the mechanisms of leukemia progression as well as to discover new drug candidates. Using the Drosophila model in combination with human cell lines, we utilise repurposing screens that aim to identify therapeutic agents, while also investigating their potential targets and their modes of action.
References:
Kúthy-Sutus, E., Kharrat, B., Gábor, E., Csordás, G., Sinka, R., Honti, V. 2022. A Novel Method for Primary Blood Cell Culturing and Selection in Drosophila melanogaster. Cells. 12,24. https://doi.org/10.3390/cells12010024
Kharrat, B., Csordás, G., Honti, V. 2022. Peeling Back the Layers of Lymph Gland Structure and Regulation. Int J Mol Sci. 23, 7767. https://doi.org/10.3390/ijms23147767
Szkalisity, Á., Piccinini, F., Beleon, A., Balassa, T., Varga, I. G., Migh, E., Molnár, C., Paavolainen, L., Timonen, S., Banerjee, I., Ikonen, E., Yamauchi, Y., Andó, I., Peltonen, J., Pietiäinen, V., Honti, V., Horváth, P. 2021. Regression plane concept for analysing continuous cellular processes with machine learning. Nat Commun. 12, 2532. https://doi.org/10.1038/s41467-021-22866-x
Balog J. Á., Honti V., Kurucz, É., Kari, B., Puskás, L. G., Andó, I., Szebeni, G. J. 2021. Immunoprofiling of Drosophila Hemocytes by Single-cell Mass Cytometry. Genomics Proteomics Bioinformatics. 19, 243-252. https://doi.org/10.1016/j.gpb.2020.06.022
Csordás, G., Gábor, E., Honti, V., 2020. There and back again: The mechanisms of differentiation and transdifferentiation in Drosophila blood cells. Dev. Biol. 469, 135-143. https://doi.org/10.1016/j.ydbio.2020.10.006
Varga, G.I.B., Csordás, G., Cinege, G., Jankovics, F., Sinka, R., Kurucz, É., Andó, I., Honti, V., 2019. Headcase is a Repressor of Lamellocyte Fate in Drosophila melanogaster. Genes 10. https://doi.org/10.3390/genes10030173
Honti, V., Csordás, G., Kurucz, É., Márkus, R., Andó, I., 2014. The cell-mediated immunity of Drosophila melanogaster: hemocyte lineages, immune compartments, microanatomy and regulation. Dev. Comp. Immunol. 42, 47–56. https://doi.org/10.1016/j.dci.2013.06.005
Honti, V., Csordás, G., Márkus, R., Kurucz, E., Jankovics, F., Andó, I., 2010. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster. Mol. Immunol. 47, 1997–2004. https://doi.org/10.1016/j.molimm.2010.04.017
Honti, V., Kurucz, É., Csordás, G., Laurinyecz, B., Márkus, R., Andó, I., 2009. In vivo detection of lamellocytes in Drosophila melanogaster. Immunol. Lett. 126, 83–84. https://doi.org/10.1016/j.imlet.2009.08.004

senior research associate

research associate

research associate

PhD student
 Viktor, HONTI
Viktor, HONTI
|
senior research associate | publications | CV |
 Erika, GÁBOR
Erika, GÁBOR
|
research associate | publications | CV |
 Zsanett, TAKÁCS
Zsanett, TAKÁCS
|
research associate | publications | CV |
 Dóra, BALOGH
Dóra, BALOGH
|
PhD student | publications | CV |