
The most dynamic component of the cytoskeleton in every eukaryotic cell is the microfilament network of linear polymers of actin subunits. Extensive research in the past decade has significantly broadened our view about the role actin plays in the life of the cell and added novel aspects to actin research. One of these new aspects is the discovery of the existence of nuclear actin which became evident only recently. In the nucleus, actin has been linked to a variety of processes including transcription and transcription regulation, RNA processing and export, chromatin organization and remodeling, DNA repair, or even nuclear envelope assembly.
One of the main areas of interest of our research group is the investigation of the biological significance of nuclear actin and the exploration of mechanisms ensuring its presence in the nucleus. In the last year, we have also started to study the role of nuclear actin in immune cell differentiation. For these experiments, the excellent model organism Drosophila melanogaster is used.
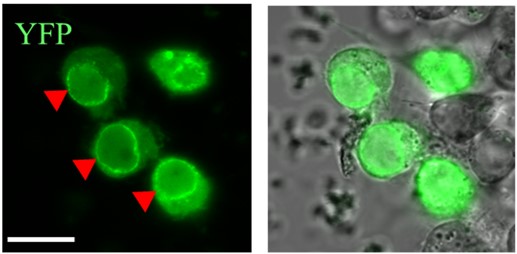
Actin binds the nuclear importin, Ketel as indicated by the green fluorescent signal.
Our research group examines also the nuclear functions of the actin-binding ERM proteins. The numerous and important functions of the actin cytoskeleton are enabled and achieved by at least 80 actin-binding proteins in the cytoplasm. Members of the actin binding Ezrin-Radixin-Moesin (ERM) protein family of vertebrates are major regulators of actin dynamics in the cell by crosslinking membrane proteins to the cortical actin network. The three paralogs are present in vertebrates, whereas other species, (e.g. Drosophila) have only one ERM gene. ERMs have pivotal role in cell adhesion, cell movements and intracellular membrane trafficking processes therefore are key players in cell polarity, morphogenesis and tumor metastasis.
By studying Moesin, Drosophila's only ERM representative, we proved that ERM proteins are also present in the nucleus. We determined the protein motives and mechanisms regulating Moesin's entry into the nucleus, and investigated the dynamics and regulation of its nuclear import. We discovered that in cooperation with the Mediator complex it participates in the regulation of the activity of heat shock genes and in mRNA export.
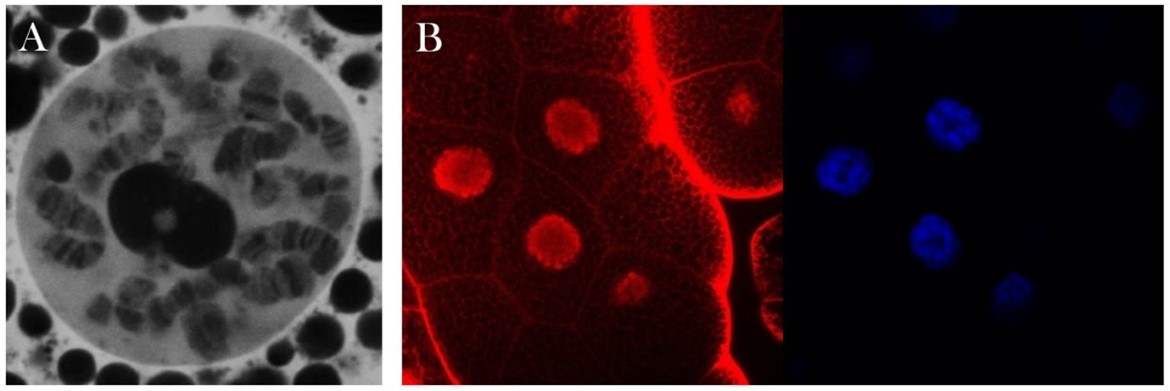
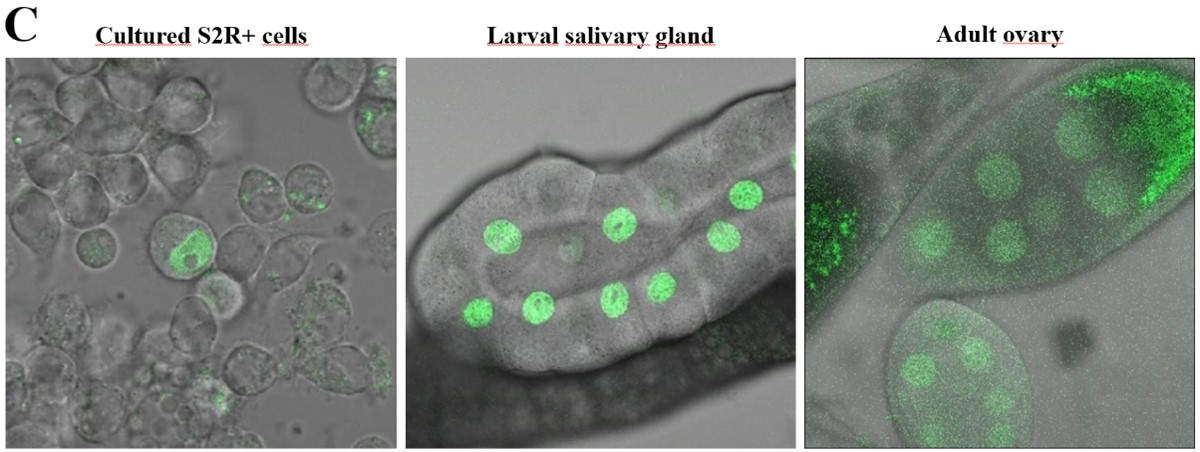
Nuclear localization of Drosophila Moesin. A) The distribution pattern of Moesin (white) in a polytenic, interphase nucleus. B) The accumulation of Moesin (red) in the nucleus (blue - DAPI). C) The interaction between Moesin and the mediator complex is indicated by the green fluorescent signal in the nucleus.
Recently, we generated a mutant in which all the cell nuclei lack or have reduced amount of Moesin. With the help of this mutant we aim to determine the biological significance of the nuclear localization of Moesin, and the problems caused by the decrease in nuclear protein level. These experiments helped us to discover that nuclear Moesin is required for the normal transcription of heat-shock induced chaperon genes.
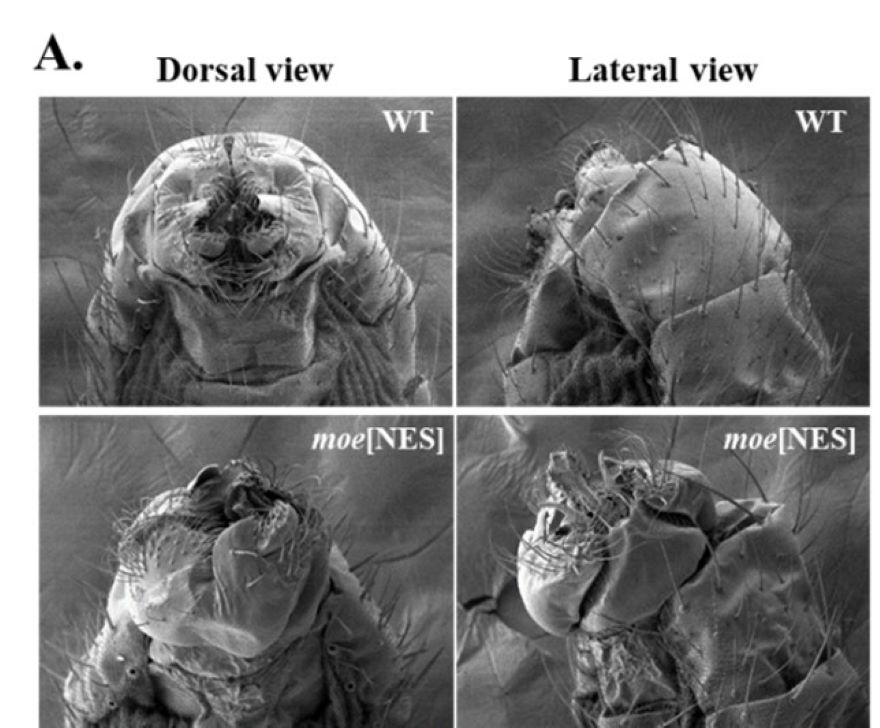
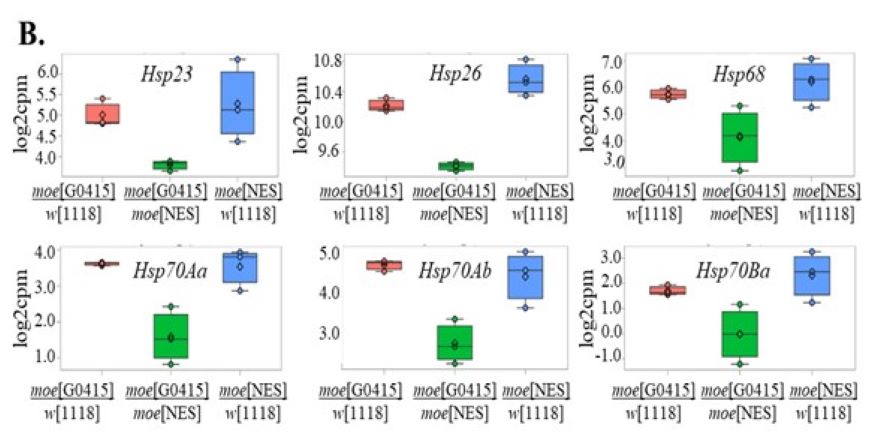
A) The genitalia are rotated in males lacking Moesin in their cell nuclei. B) The expression of heat shock chaperons is decreased (green) in the mutant animals.
To study the role ERMs play in the nucleus, we use advanced light microscopy, biochemical and molecular biological methods, and the fruit-fly and cultured Drosophila cell lines.

senior research associate

research associate

research associate

junior research associate

administrator expert
 Péter, VILMOS
Péter, VILMOS
|
senior research associate | publications | CV |
 Ildikó, KRISTÓ
Ildikó, KRISTÓ
|
research associate | publications | CV |
 Anikó, SZABÓ
Anikó, SZABÓ
|
research associate | publications | CV |
 Zoltán, KOVÁCS
Zoltán, KOVÁCS
|
junior research associate | publications | CV |
 Csilla, ABONYI
Csilla, ABONYI
|
administrator expert | publications |