
Brief introduction
Research in our group is focused on the understanding structural mechanisms of protein function and protein - small molecule interactions. Potential drug target G protein-coupled receptors are investigated by in silico modeling techniques and in vitro experimental methods to obtain structure - activity/function relationships. The group is also engaged in the preparation of small molecules, peptides and proteins enriched in chemical information, i.e. stable isotopes, unnatural building blocks, fluorophores, radioactive labels. Furthermore, protein modification strategies are also developed for the preparation of membrane associated proteins.
Ongoing Projects
1. The structural mechanism of G protein-coupled receptor activation (A. Borics)
G protein-coupled receptors (GPCRs) represent one of the largest protein superfamily of the human genome and participate in crucial physiological functions. GPCRs are involved in many diseases, hence approximately one third of all prescription pharmaceuticals target members of this receptor family. High-resolution structures of active and inactive state GPCRs are available but the process of transition between these states is poorly understood. We intend to identify the role of ligand-receptor interactions with respect to the electrostatic equilibrium in order to reveal general aspects of the function of this receptor family. Our investigations are based on the introduction of a new perspective, focusing on the charge distribution within the receptor. Our most recent, unbiased, atomistic molecular dynamics simulations of the m-opioid receptor in a physiological environment revealed that external stimulus could be propagated to the intracellular surface of the receptor through subtle, concerted movements of highly conserved polar amino acid side chains along the 7th transmembrane helix. We suggest that the initiation event of GPCR activation is the shift of macroscopic polarization between the ortho- and allosteric binding pockets and the intracellular G protein-binding interface. Extension of this study to further class A GPCRs, such as the b2-adrenergic and type I cannabinoid receptors, provided further support for the above hypothesis. A signaling channel, consisting of highly conserved polar motifs, connecting the ligand binding pocket with the intracellular G protein binding surface was identified in all three of the aforementioned receptors. Modifications of the constituent amino acids of this polar signaling channel led to the loss of ligand binding and G protein activation in the m-opioid receptor, as indicated by recent site directed mutagenesis and in vitro pharmacological studies. Currently, specific ligand-receptor interactions responsible for receptor activation are investigated, utilizing de novo interaction specific ligands, mutant receptors, in vitro functional assays, mixed quantum mechanics/molecular mechanics calculations (QM/MM) and saturation transfer triple difference (STTD) 1H-NMR spectroscopic measurements.
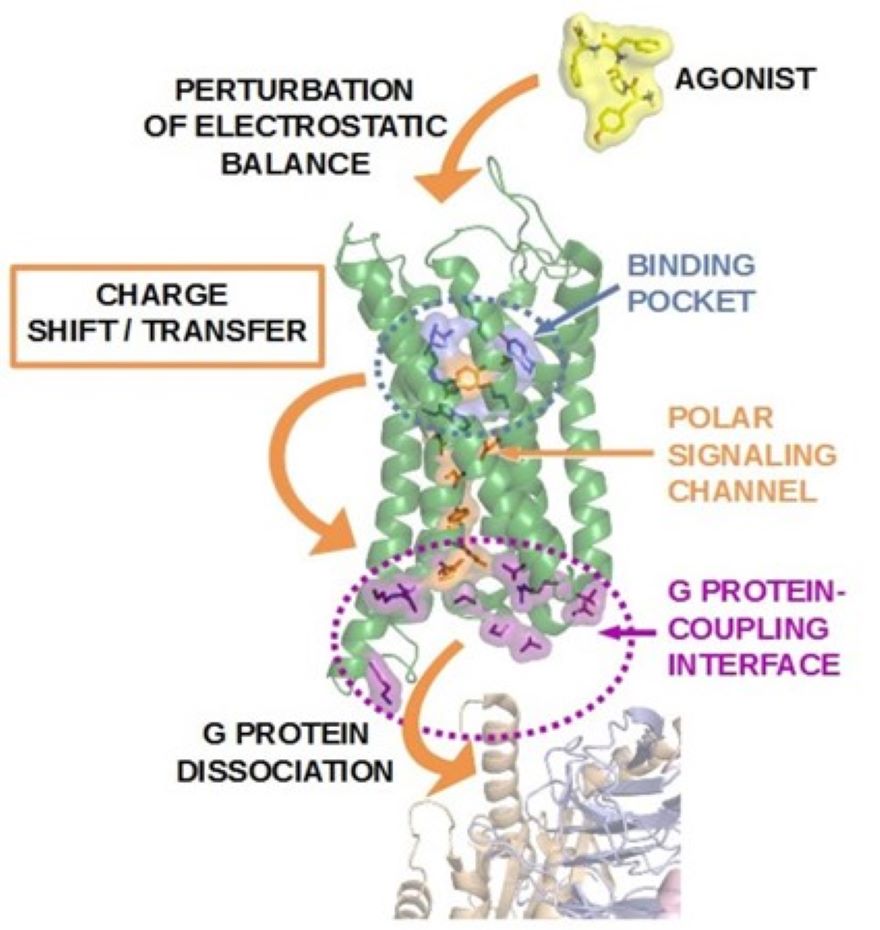
Figure 1. Putative structural mechanism of G protein-coupled receptor activation
2. Development of type I cannabinoid receptor negative allosteric modulators against illicit synthetic cannabinoid toxicity (Sz. Dvorácskó)
The endocannabinoid system (ECS) is involved in numerous physiological and pathological processes in the central nervous system and in the periphery. Synthetic compounds that interact with the cannabinoid receptors (CBRs) or modulate the EC signaling have therapeutic potential in the treatment of neurological diseases, psychiatric disorders, neuropathic pain and obesity. However, despite the useful effects of plant-derived and synthetic CBR ligands, their psychoactive effects limit their clinical applications. Furthermore, there are highly potent synthetic CB1R agonists among illicit drugs that are dangerous, occasionally lethal compounds worldwide with distinct, mostly unknown clinical effects and with diverse pharmacodynamics and pharmacokinetics. At present there is no specific antidote for the intoxication of such synthetic cannabinoids. Recently, a unique family of endogenous peptides (hemopressins or pepcans) that are structurally distinct from the endogenous lipid cannabinoids have been described to be CBR ligands. Among them, pepcan-12 is the most abundant peptide that was found to be able to modulate anxiety, food intake, intestinal motility and analgesia in rodents with little or no psychoactive side effect. According to our and others’ in vitro results pepcan-12 is a negative allosteric modulator of CB1R, therefore we hypothesize that pepcan-12 and its synthetic analogues are excellent lead compounds for targeting the CBRs to develop peptide therapeutics without psychoactive side effects. In this project, the preparation of modified pepcans will be performed followed by their detailed pharmacological characterization at molecular and behavioural levels.
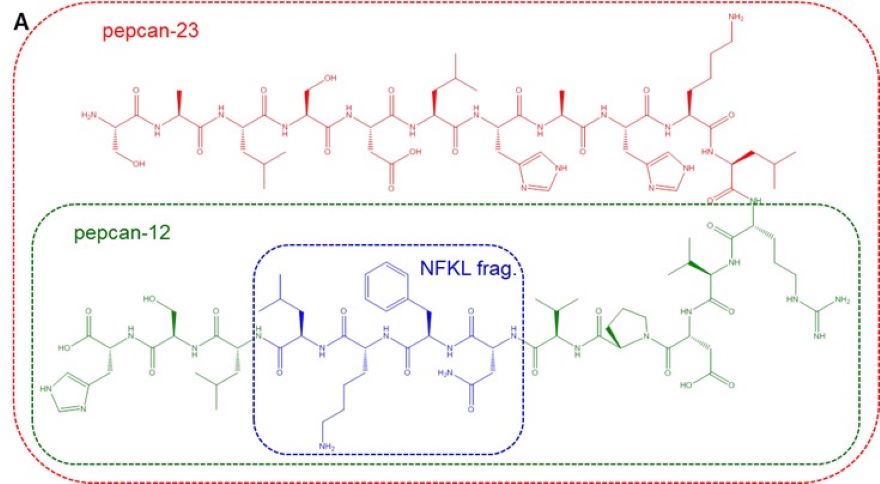
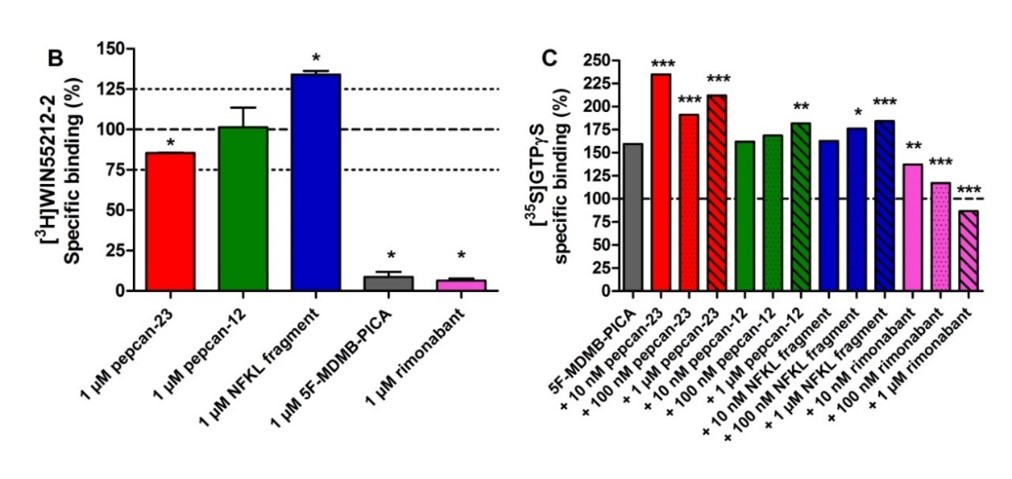
Figure 2. The modulation effects of pepcans and hemopressins: (A) structure (B) interaction with cannabinoid 1 receptors and (C) functional activity
3. Membrane associated proteins (Cs. Tömböly)
The exogenous introduction of semisynthetic lipoproteins into the plasma membrane is a cell surface modification method that offers strict control over the composition of the engineered cell membrane. Therefore, it is an accurate approach for the investigation of membrane protein dynamics, for visualizing the cellular traffic of membrane proteins, and for improving the efficacy of biologically active proteins and cell based therapeutics. In order to deliver proteins into the plasma membrane with synthetic anchor molecules, we have developed a mild method and demonstrated that a cholesterol anchor and the attached protein had been incorporated into the membrane of live cells in stoichiometric ratio. Our cholesterol anchor is water soluble, non-toxic and appropriate for live cell cultures without membrane perturbing detergents. Additional advanced feature of our anchor is a fluorescent or radioactive reporter in the headgroup that facilitates the direct imaging of the attached protein. Since cholesterol favors the interaction with sphingolipids, it has the potential to target the attached protein to lipid rafts. The usefulness of our anchors was demonstrated in a proof-of-concept study where the red fluorescent protein mCherry and the full length prion protein were anchored to SHSY-5Y neuroblastoma cell membranes. Atomic details of the distribution and orientation of the GPI anchor mimetic cholesterol derivatives in a model lipid bilayer were obtained by theoretical methods.
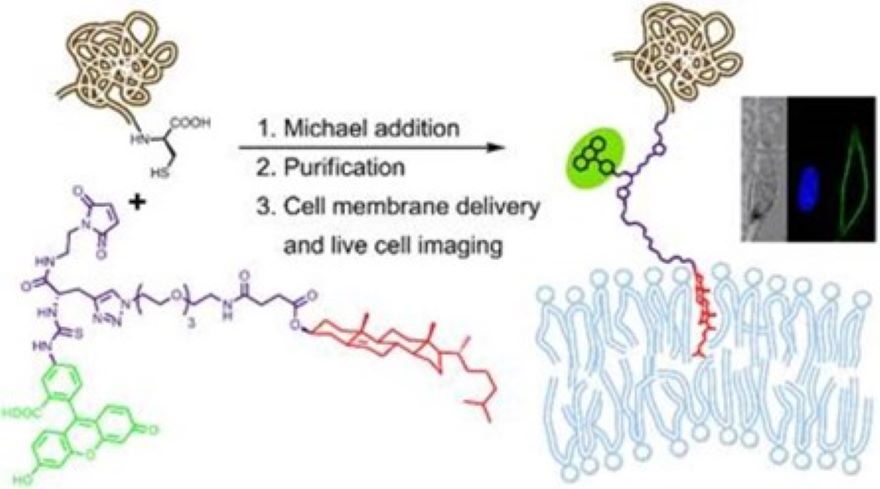
Figure 3. Synthetic approach for the membrane anchoring and fluorescent labeling of semisynthetic proteins.
4. mRNA vaccine lipids (Cs. Tömböly)
Vaccinating and other immunotherapic objectives are achivable through cell transfection with messenger RNA (mRNA) lipid nanoparticle (LNP) formulations. One pivotal component of the LNPs are ionisable lipids which are amphiphilic molecules comprise a fatty acid chain connected to an ionisable head group. In our lab, in collaboration with the National Laboratory of Biotechnology we aim to synthetise a modular ionisable lipid library for the production and optimisation of a vaccine against african swine fever virus (ASFV). The modularity of our library enables the adjustment of the pharmacologic properties of different mRNA-LNP formulations for different purposes.
Selected publications
Sarkar A, Mitra A, Borics A. All-Atom Molecular Dynamics Simulations Indicated the Involvement of a Conserved Polar Signaling Channel in the Activation Mechanism of the Type I Cannabinoid Receptor. Int J Mol Sci. 2023, 24, 4232. doi: 10.3390/ijms24044232.
Dvorácskó Sz, Herrerias A, Oliverio A, Bhattacharjee P, Pommerolle L, Liu Z, Feng D, Lee Y-S, Hassan SA, Godlewski G, Cinar R, Iyer MR. Cannabinoformins: Designing biguanide-embedded, orally available, peripherally selective cannabinoid-1 receptor antagonists for metabolic syndrome disorders, J. Med. Chem., 2023, 66, 11985-004. doi:10.1021/acs.jmedchem.3c00599.
Dvorácskó Sz, Dimmito MP, Sebastiani J, La Regina G, Silvestri R, Pieretti S, Stefanucci A, Tömböly Cs, Mollica A. Rimonabant-Based Compounds Bearing Hydrophobic Amino Acid Derivatives as Cannabinoid Receptor Subtype 1 Ligands. ACS Med Chem Lett, 2023, 9, 479-486. doi: 10.1021/acsmedchemlett.3c00024
Mitra A, Sarkar A, Borics A. Universal Properties and Specificities of the β2-Adrenergic Receptor-Gs Protein Complex Activation Mechanism Revealed by All-Atom Molecular Dynamics Simulations. Int J Mol Sci. 2021, 22, 10423. doi: 10.3390/ijms221910423.
Mitra A, Sarkar A, Szabó MR, Borics A. Correlated Motions of Conserved Polar Motifs Lay out a Plausible Mechanism of G Protein-Coupled Receptor Activation. Biomolecules. 2021, 11, 670. doi: 10.3390/biom11050670.
Dvorácskó Sz, Keresztes A, Mollica A, Stefanucci A, Macedonio G, Pieretti S, Zádor F, Walter F, Deli M, Kékesi G, Bánki L, Tuboly G, Horváth Gy, Tömböly Cs. Preparation of bivalent agonists for targeting the mu opioid and cannabinoid receptors. Eur. J. Med. Chem. 2019, 178, 571-88. doi: 10.1016/j.ejmech.2019.05.037
Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak GW, McLaughlin JP, Majumdar S. Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2. J Med Chem. 2016, 59, 8381-97. doi: 10.1021/acs.jmedchem.6b00748.
Schäfer B, Orbán E, Borics A, Huszár K, Nyeste A, Welker E, Tömböly C. Preparation of semisynthetic lipoproteins with fluorescent cholesterol anchor and their introduction to the cell membrane with minimal disruption of the membrane. Bioconjug Chem. 2013, 24, 1684-97. doi: 10.1021/bc4002135.
Tóth G, Borics A. Closing of the flaps of HIV-1 protease induced by substrate binding: a model of a flap closing mechanism in retroviral aspartic proteases. Biochemistry. 2006, 45, 6606-14. doi: 10.1021/bi060188k.
Patents
Iyer MR, Dvorácskó Sz, Bhattacharjee P, Cinar R, Kunos G. PCT Patent Application No. PCT/U2023/014846 Cannabinoid receptor modulating compounds)
Mollica A, Macedonio G, Stefanucci A, Dvorácskó Sz, Tömböly Cs. Indazole derivatives as modulators of the cannabinoid system. EP18170728 (pending)
Methodolgical portfolio
Wet chemistry
Solid and solution phase peptide synthesis (e.g. hemopressins, opioid peptides)
Design, and synthesis of organic compounds (e.g. synthetic cannabinoids)
Radiolabeling of bioactive compounds
Analytical and preparative separation techniques
Proteolytic degradation assay
Experience in forensic techniques focusing on illicit synthetic cannabinoids
In silico chemistry
Molecular dynamics (conventional, replica exchange, simulated annealing, steered, folding, etc.)
Molecular docking (protein-small molecule, protein-protein)
Force field parametrization of small organic molecules and non-natural amino acids
Three-dimensional structure prediction of proteins
Quantum chemical calculations
Vibrational spectrum calculations
In vitro pharmacology
Animal tissue preparation and cell culture techniques
Radioligand receptor binding assays
Radioligand [35S]GTPγS binding functional assay
TR-FRET functional assay (cAMP)
Chemiluminescent functional assay (β-arrestin-2 recruitment)
ELISA (AMPK activity) assay.
Radioligand NOS activity assay
In vivo pharmacology
Drug treatment techniques
Pharmacokinetic and tissue distribution studies
Functional assays
Behavioral experiments (elevated plus maze assay, alcohol drinking models)
’Tetrad test’ (locomotion, body temperature, hot plate analgesia, catalepsy)

senior research associate

tudományos munkatárs

PhD student

research assistant

laboratory assistant

senior research associate (currently on sabbatical)
 BORICS, Attila
BORICS, Attila
|
senior research associate | publications | CV |
 DVORÁCSKÓ, Szabolcs
DVORÁCSKÓ, Szabolcs
|
tudományos munkatárs | publications | CV |
 SARKAR, Arijit
SARKAR, Arijit
|
PhD student | publications | CV |
 KÖDMÖN, Ádám
KÖDMÖN, Ádám
|
research assistant | CV | |
 TÓTHNÉ PAPP, Éva
TÓTHNÉ PAPP, Éva
|
laboratory assistant | ||
 TÖMBÖLY, Csaba
TÖMBÖLY, Csaba
|
senior research associate (currently on sabbatical) | publications | CV |