
At the Cellular Imaging Laboratory, we are strongly committed to the development and application of
advanced imaging, fluorescent labeling and biochemical techniques and approaches that will enable
us to understand the complex organization within and between cells. Our recent achievements
include (i) the application of the fast and robust ethynyldeoxyuridine-based replication assay for
plant cells, (ii) the discovery of novel blue fluorescent dyes for in vivo detection, and (iii) 3D analysis
of lipid droplets and high efficiency oligonucleotide-directed gene editing monitored by mutant GFP
and fluorescence imaging methods.
Our modern imaging center is equipped with confocal laser scanning and spinning disc microscopes,
fluorescence and stereo microscopes, a laser microdissection microscope, and powerful image
analysis computers with imaging software. With these modern microscopes we can perform protein
localization and mobility analyses, three dimensional, time course dynamic analyses of live cells,
tissues and organisms. Thanks to the new imaging techniques and the development of new
fluorescent dyes and proteins, today’s biological and medical research has increasingly become
dependent on microscopy and image analysis. It is now possible to specifically label virtually any
molecule and directly probe its function in live cells by light microscopy.
This ability to visualize the dynamics of proteins in vesicles, organelles, cells and tissue has begun to
provide new insights into how cells function in health and disease. Such work yields unique
mechanistic insight by directly illustrating the complex spatial-temporal dynamics of fundamental
cellular processes such as mitosis, morphogenesis, polarization, embryonic development, membrane
trafficking and cytoskeleton dynamics. Many of these processes are highly dynamic and are
challenging to image by traditional means. In this aim, we are strongly committed to the
development and application of optical imaging, fluorescent labeling and biochemical techniques
that will enable us and others to understand the complex organization within and between cells.
Labeling, detection and quantification of cells in the S-phase (DNA synthesis) of cell cycle progression
are crucial in characterizing the cellular responses to various treatments and genetic modifications.
Bromo-deoxyuridine (BrdU) labeling of cells followed by antibody staining is the standard method for
detecting cells in the S-phase. Antibody detection of BrdU involves harsh treatments or nuclease
digestion to facilitate epitope access. Moreover, in plants cells, cell wall digestion is also necessary.
These steps could interfere with cellular morphology, and are time-consuming. We have optimized
the recently developed ethynyl-deoxyuridine (EdU) method on plant cell cultures and seed-derived
roots as well as on isolated plant nuclei using confocal laser scanning microscopy and flow cytometry
(Kotogany et al., 2010, Ayaydin et al., 2011, Kuntam and Ayaydin, 2015)
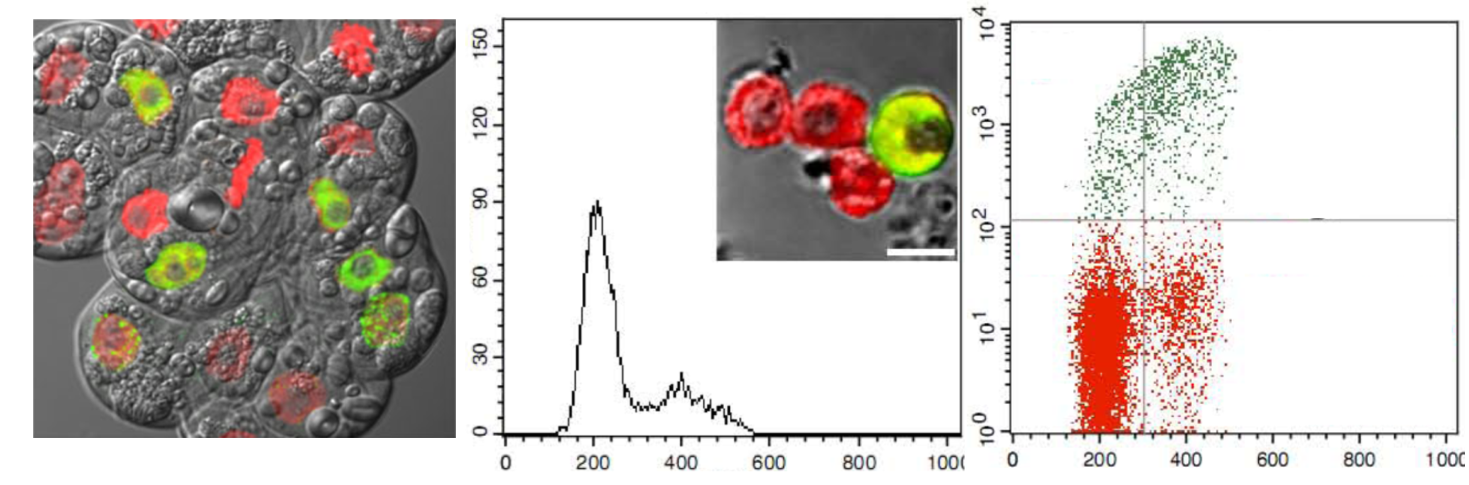
Microscopy and cytometry application of EdU-based replication assay
Plant lipid droplets (LDs), similar to their yeast and mammalian counterparts, are highly dynamic.
Their proteomic analysis has revealed that a host of proteins reside on their surface with varying
composition under different conditions. However, a lot remains to be uncovered in the area of LD
protein and lipid composition as well as LD transport, mechanism of protein targeting, assembly and
regulation. Live cell analysis is thus required to unravel the dynamic regulation of this important
organelle. We have recently reported the development and characterization of novel fluorochromes
as markers for LDs in living plant cells that emit in the blue range hence presenting flexibility during
multicolor imaging. (Kuntam et al., 2015)
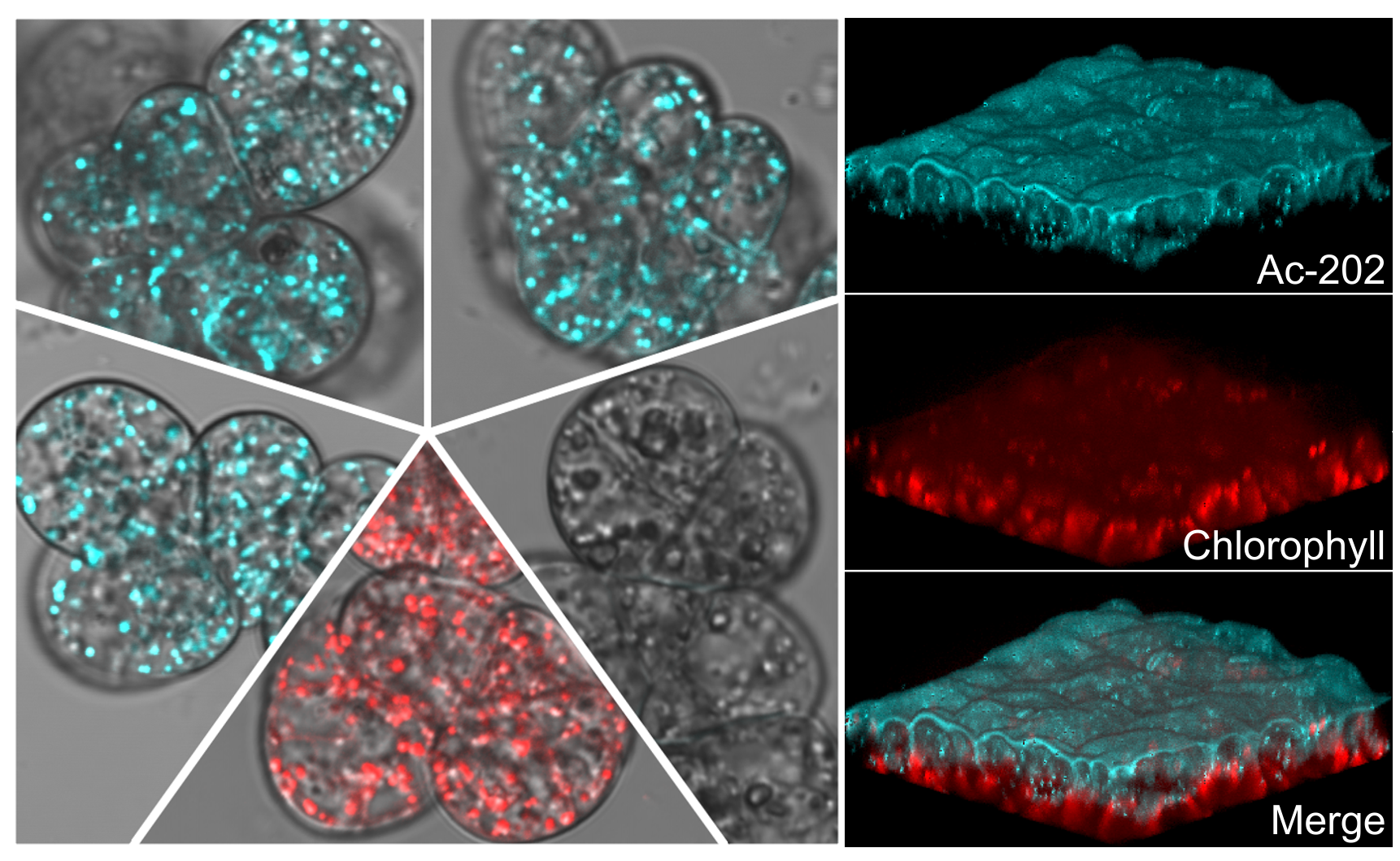
Discovery of novel fluorescent dyes for plant lipid droplet imaging
Targeted genome editing has been developed as an alternative to classical mutation breeding and
transgenic (GMO) methods to improve crop plants. The Oligonucleotide Directed Mutagenesis (ODM)
as Targeted Nucleotide Exchange (TNE) by single stranded DNA oligonucleotides (SDOs) attracts
special attention for use in both basic science and plant breeding. On the other hand, one of the
major limitations of this technique is the low frequency of TNE events. Using a mutant version of
green fluorescent protein and chromatin modifying agents, recently, in collaboration with Prof.
Dénes Dudits (Institute of Plant Biology, HUN-REN BRC, Szeged), we have achieved significantly increased
frequency of TNE on cultured maize cells. We predict our results will increase the use and
applicability of this new gene editing technique, which in turn may lead to crops with improved
characteristics not imparted by introduction of foreign DNA sequences. (Tiricz et al, 2015)
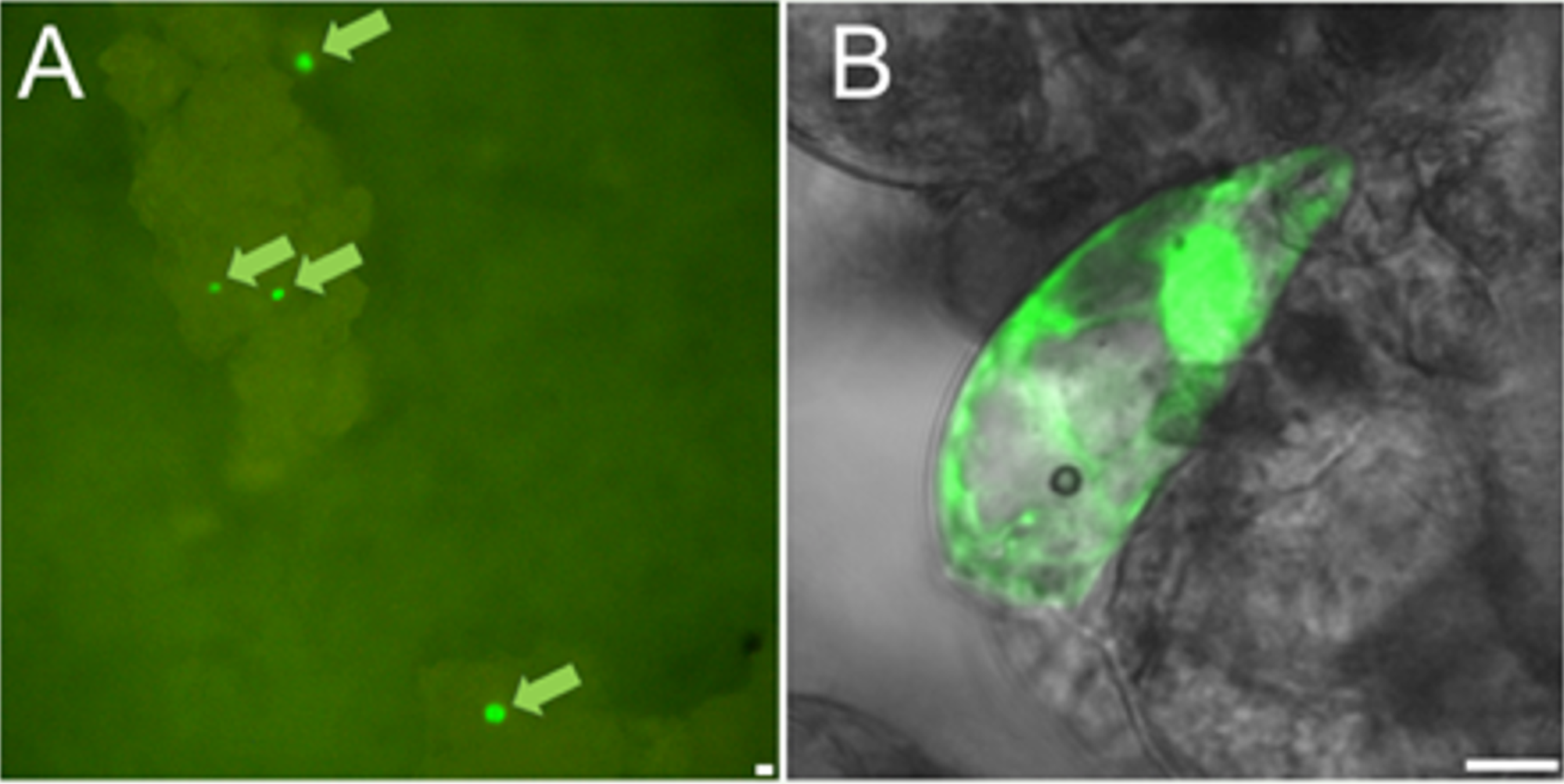
Restoration of GFP fluorescence by targeted nucleotide exchange
Industrial activities necessitate the production of toxic chemicals for various purposes. These
compounds are of different hazard levels, some being highly toxic. They are not only present in our
surroundings during their manufacturing but also during their storage and transportation and can
pose significant health hazard via respiratory pathway, contact through skin or by ingestion. Thus,
the ability to monitor continuously their environmental levels with high sensitivity and accuracy is of
key importance. In our recently initiated bilateral project (2019, NKFI TR-NN_17 Hungary-Turkey), we
aim to develop a highly sensitive biosensor based toxic chemical detection platform and a flying
device (drone) capable of monitoring these chemicals instantaneously, continuously over a
prolonged time and over a large geographical area. Such a device will be outperforming the
sensitivity and speed of detection of currently available devices, by being highly sensitive and
permitting instantaneous data collection and transmission at the same time of sensing. In our
laboratory, we have already successfully miniaturized a fluorescence detection system ready to be
integrated into a drone for sensitive detection of toxic chemicals using biosensor bacteria.
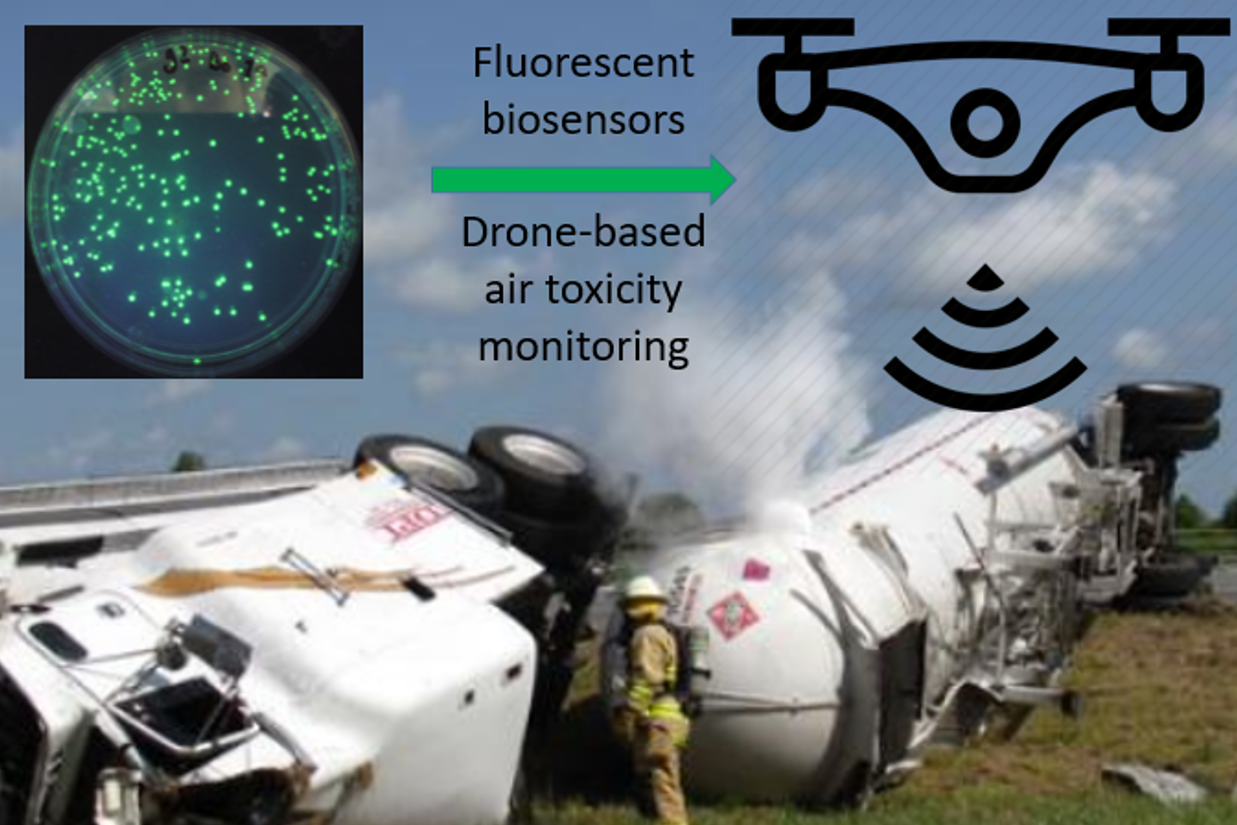
Drone-integrated pollution monitoring using fluorescent biosensor bacteria
DP microscopy provides 2D or 3D maps of the most important physical parameters characteristic of
the anisotropic molecular architecture of specimens. This is achieved by measuring different
spectropolarimetric quantities, pixel-by-pixel or voxel-by-voxel. Our knowledge about the anisotropic
architecture of microscopic specimens is very far from being complete, especially in biology.
Numerous examples demonstrate that various forms of anisotropy are present in highly organized
molecular assemblies, organelles, cells and tissues. Detailed characterization requires
microspectropolarimetric tools, which justifes our R&D efforts to combine the techniques of
advanced microscopy and spectropolarimetry,
Our DP-LSMs have been used in a number of collaborative works. The birefringence of chloroplast
thylakoid membranes revealed unexpectedly large local LB values – leading to the discovery that
anisotropic structures with high LBs are amenable for micromanipulation with polarized beam of
laser tweezers. We have imaged the domain structure of human lymphocyte cells – these results are
of interest with regards to immunology. Our FDLD-imaging of actinbased macroassemblies led to the
recognition of the role of some proteins in organizing specific cellular (O-ring canal) structures –
these data advanced our understanding of processes of cell development. High-precision FDLD
measurements were performed on plant tissues in order to better understand the architecture of
cellulose in cell walls upon environmental or mechanical stresses – this technique is suitable to
monitor the processes of enzymatic or chemicallyinduced decomposition of cellulose, a rich energy
source. The stability of human amyloids was indicated to depend on the supercoiling of protein
aggregates – this is of interest in the research of neurodegenerative diseases. By combining
microscopic and macroscopic DP techniques, we revealed the orientation of pigment dipoles in self-
assembled nanorods of porphyrin derivatives – this type of structures might be utilized in
photocatalytic systems.
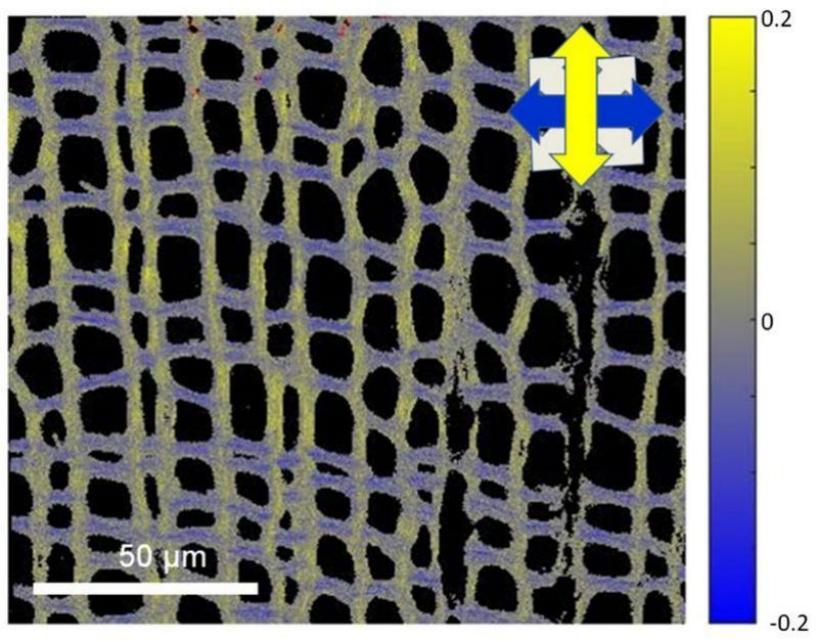
FDLD image of Ginkgo biloba tissue recorded using our DP-equipped RCM
Ayaydin, F., Vissi, E., Meszaros, T., Miskolczi, P., Kovacs, I., Feher, A., Dombradi, V., Erdodi, F., Gergely, P. and Dudits, D. (2000). Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M transition and early mitotic microtubule organization. Plant J. 23(1):85-96.
Ayaydin, F. and Dasso, M. (2004). Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol. Biol. Cell 15(12):5208-5218.
Mukhopadhyay D, Ayaydin F., Kolli N., Tan S.H., Anan T., Kametaka A., Azuma Y., Wilkinson K.D., Dasso M. (2006). SUSP1 antagonizes formation of highly SUMO2/3-conjugated species, J Cell Biol. 174: 939-949.
Kotogány, E., Dudits, D., Horváth, V.G. and Ayaydin F. (2010). A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods (6:5)
Ayaydin, F., Kotogány E., Ábrahám E and Horváth V.G. (2011). Synchronization of Medicago sativa Cell Suspension Culture. Methods Mol Biol. 761:227-238.
Fazakas C., Wilhelm I., Nagyoszi P., Farkas A.E., Haskó J., Molnar J., Bauer H., Bauer H.C., Ayaydin F., Dung N.T.K., Siklós L., Krizbai I.A. (2011). Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases, PLOS ONE 6: (6) e20758.
Rigo G., Ayaydin F., Tietz O., Zsigmond L., Kovacs H., Pay A., Salchert K., Darula Z., Medzihradszky K.F., Szabados L., Palme K., Koncz C., Cseplo A. (2013). Inactivation of Plasma Membrane-Localized CDPK-RELATED KINASE5 Decelerates PIN2 Exocytosis and Root Gravitropic Response in Arabidopsis. Plant Cell 25:1592-1608.
Gungor B., Gombos I., Crul T., Ayaydin F., Szabo L., Torok Z., Mates L., Vigh L., Horvath I. (2014). Rac1 participates in thermally induced alterations of the cytoskeleton, cell morphology and lipid rafts, and regulates the expression of heat shock proteins in B16F10 melanoma cells. PLOS ONE 9:(2) p. e89136.
Steinbach G, Pawlak K, Pomozi I, Tóth EA, Molnár A, Matkó J, Garab G (2014) Mapping microscopic order in plant and mammalian cells and tissues: novel differential polarization attachment for new generation confocal microscopes (DP-LSM). Methods and Applications in Fluorescence 2: 015005 (9pp)
Kuntam S., Puskás L.G., Ayaydin F. (2015). Characterization of a new class of blue-fluorescent lipid droplet markers for live-cell imaging in plants. Plant Cell Rep. 34:655-665.
Kuntam S. and Ayaydin F.(2015) Detection of S-phase of cell division cycle in plant cells and tissues by using 5-ethynyl-2’-deoxyuridine (EdU) in Plant Microtechniques: Methods and Protocols. Eds. Yeung C.T.E., Stasolla C, Sumner M.J., Huang B.Q. Springer, pp. 311-322.
Horvath B., Domonkos A., Kereszt A., Szucs A., Abraham E., Ayaydin F., Boka K., Chen Y., Chen R., Murray J.D., Udvardi M.K., Kondorosi E., Kalo P. (2015) Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc. Natl. Acad. Sci. USA 112:15232-15237.
Steinbach G, Schubert F, Kaňa R (2015) Cryo-imaging of photosystems and phycobilisomes in Anabaena sp. PCC 7120 cells. Journal of Photochemistry and Photobiology B: Biology, 152: 395–399
Dudits D., Török K., Cseri A., Paul K., Nagy A.V., Nagy B., Sass L., Ferenc G., Vankova R., Dobrev P., Vass I., Ayaydin F. (2016) Response of Organ Structure and Physiology to Autotetraploidization in Early Development of Energy Willow Salix viminalis. Plant Physiol. 170:1504-1523.
Steinbach G, Kaňa R (2016) Automated microscopy: macro language controlling a confocal microscope and its external illumination – adaptation for photosynthetic organisms. Microscopy and Microanalysis, 22: 258–263
Tiricz H., Nagy B., Ferenc G., Török K., Nagy I., Dudits D. and Ayaydin F. (2017) Relaxed chromatin induced by histone deacetylase inhibitors improves the oligonucleotide-directed gene editing in plant cells. J Plant Res 131(1):179-189.
Fodor E and Ayaydin F. (2018) Fluorescent probes and live imaging of plant cells. “Advances in Plant Ecophysiology Techniques” Eds: Reigosa MJ, Sánchez-Moreiras A. Publisher: Springer Nature pp. 241-251
Steinbach G, Nagy N, Sipka G, Manders E, Garab G, Zimanyi L (2019) Re-scan confocal microscope modified for anisotropy imaging – as a part of a differential polarization system. European Biophysics Journal 48: 457–463. 2019
Strašková A, Steinbach G, Kotabová E, Komenda J, Tichý M, Kaňa R (2019) Pigment-protein complexes are organized into stable microdomains in cyanobacterial thylakoids. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1860, 148053
Kana R, Steinbach G, Sobotka R, 1 , Vámosi G, Komenda J (2021) Fast Diffusion of the Unassembled PetC1-GFP Protein in the Cyanobacterial Thylakoid Membrane. Life 11: 15, 2021
Bernát, G., Zavřel, T., Kotabová, E., Kovács, L., Steinbach, G., Vörös, L., Prášil, O., Somogyi, B., Tóth, V. R. (2021) Photomorphogenesis in the Picocyanobacterium Cyanobium gracile Includes Increased Phycobilisome Abundance Under Blue Light, Phycobilisome Decoupling Under Near Far-Red Light, and Wavelength-Specific Photoprotective Strategies. FRONTIERS IN PLANT SCIENCE 12 Paper: 612302 , 16 p. (2021)
Pleckaitis, M., Habach, F., Kontenis, L., Steinbach, G., Jarockyte, G., Kalnaityte, A., Domonkos, I., Akhtar, P., Alizadeh, M., Bagdonas, S., Karabanovas, V., Garab, G., Rotomskis, R., Barzda, V. (2022) Structure and principles of self-assembly of giant "sea urchin" type sulfonatophenyl porphine aggregates. NANO RESEARCH 15 : 6 pp. 5527-5537. , 11 p. (2022)
Dudits, D., Cseri, A., Török, K., Vankova, R., Dobrev, P. I., Sass, L., Steinbach, G., Kelemen-Valkony, I., Zombori, Z., Ferenc, G., Ayaydin, F. (2023) Manifestation of Triploid Heterosis in the Root System after Crossing Diploid and Autotetraploid Energy Willow Plants. GENES 14 : 10 p. 1929