
The neurovascular unit (NVU) plays a key role in the maintenance of the homeostasis of the central nervous system (CNS). Its most important cellular components are cerebral endothelial cells (interconnected by a continuous line of tight and adherens junctions), pericytes and astrocytes.
The main role of the NVU is the formation of a functionally active interface between the circulation and the CNS called the blood-brain barrier (BBB) and the regulation of blood supply according to neuronal demand (neurovascular coupling).
Due to its complex functions, the NVU plays an important role in clinical practice. First, the NVU is involved in the mechanism of a large number of CNS diseases like stroke, brain trauma, tumors, as well as neurodegenerative disorders, and is crucially involved in age-related processes. Second, due to the relative impermeability of brain capillaries, many drugs are unable to reach the CNS in therapeutically relevant concentration, making the BBB one of the major impediments in the treatment of CNS disorders.
Our research is focused on the elucidation of the molecular mechanisms regulating NVU functions under physiological and pathological conditions. By using different in vitro models of the BBB and in vivo systems, presently we are investigating the role of the NVU in age-related processes and and brain metastasis formation.
In vitro models
To directly investigate the molecular mechanisms regulating NVU functions, we are using an in vitro model based on the culture of cerebral endothelial cells and other cells (pericytes, astrocytes). The model can be used in basic science and applied research as well for the study of the transport of different drugs through the BBB.
Our methods of investigation include different biochemistry and molecular biology techniques, immunofluorescence and functional tests of barrier properties.

Figure 1. A: Cellular structure of the NVU. B: In vitro model of the NVU
In vivo models and advanced microscopic techniques
We are using two-photon microscopy and transgenic animals to reveal morphological and functional changes of the NVU under physiological and pathological circumstances in vivo. Besides, superresolution microscopy (STED) is also available in our laboratory to monitor changes at the submicroscopic level.
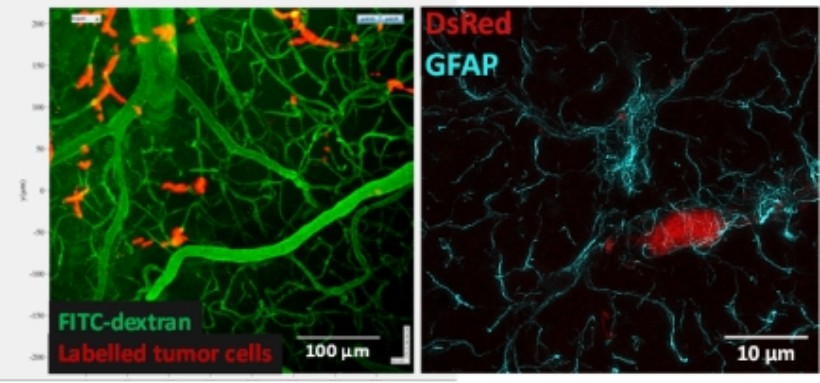
Figure 2. A: Two-photon image of the brain of a living animal. B: STED image of astrocytes (cyan) and pericytes (red)
Role of the NVU in inflammatory and age-related processes
Inflammatory processes are associated with a large number of physiological and pathological conditions of the CNS including stroke, brain trauma, tumors, neurodegenerative disorders, as well as aging. Our studies are focused on the investigation of pattern recognition receptors and inflammasome activation. Pericytes may play a key role in inflammatory and age-related processes of the NVU. Therefore, our investigations include in vitro and in vivo monitoring of brain pericyte functions and contractility during aging-related processes.
Brain capillaries are particularly affected during aging, while the regenerative capacity of the endothelium is extremely limited. Therefore, we pay special attention to explore the therapeutic potential of endothelial precursor cells to alleviate age-induced capillary and consequently cerebral dysfunctions.
Interaction of metastatic cells with the NVU
Despite the potential obstacle represented by the BBB for extravasating malignant cells, metastases – originating primarily from lung cancer, breast cancer and melanoma – are more frequent than primary tumours in the CNS. The brain environment may be very hostile to metastatic cells; however, tumour cells which can adapt to the specific requirements of the brain can exploit several advantages, including dense vascularization and shielding against the immune system and drugs.
In the framework of the project, we are studying mechanisms related to endothelial cells, pericytes and glial cells during two specific steps of brain metastasis formation, transmigration of the tumour cells through cerebral capillaries and survival of the metastatic cells in the brain environment. We are dedicated to understanding the complex interaction between tumour cells and host cells in the brain, which is essential for the identification of new therapeutic targets in this devastating disease.
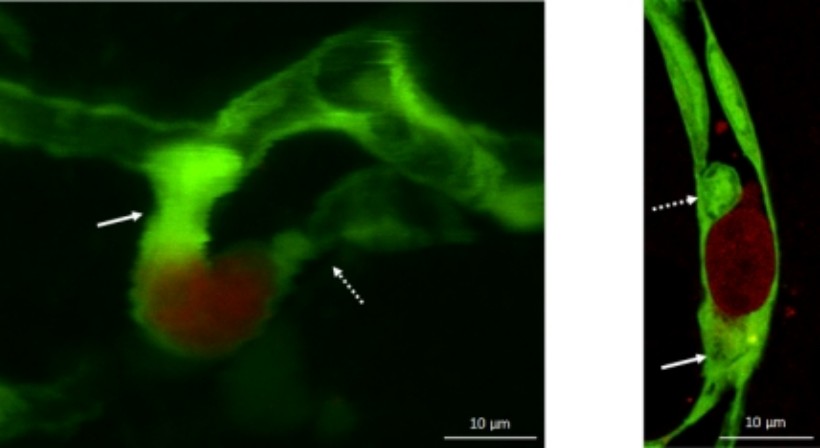
Figure 3. Metastatic cells (red) in the initial stage of the transmigration process (green: endothelial cells). Arrows indicate capillary constriction and endothelial plugs
-Ádám Mészáros*, Kinga Molnár*, Csilla Fazakas, Bernát Nógrádi, Adél Lüvi, Tamás Dudás, László Tiszlavicz, Attila Elek Farkas, István Adorján Krizbai#, Imola Wilhelm#. Inflammasome activation in peritumoral astrocytes is a key player in breast cancer brain metastasis development. Acta Neuropathol Commun. 2023 Sep 25;11(1):155. doi: 10.1186/s40478-023-01646-2. (IF2022: 7,1). D1*első szerzők, #levelező szerzők
-Molnár K*, Nógrádi B,*, Kristóf R, Mészáros Á, Pajer K, Siklós L, Nógrádi A, Wilhelm I# and Krizbai IA# Motoneuronal inflammasome activation triggers excessive neuroinflammation and impedes regeneration after sciatic nerve injury. J Neuroinflammation. 2022 Mar 19;19(1):68. doi: 10.1186/s12974-022-02427-9. (IF2022: 9,3) D1. *első szerzők, #levelező szerzők
-Menyhárt Á, Frank R, Farkas AE, Süle Z, Varga VÉ, Nyúl-Tóth Á, Meiller A, Ivánkovits-Kiss O, Lemale CL, Szabó Í, Tóth R, Zölei-Szénási D, Woitzik J, Marinesco S, Krizbai IA, Bari F, Dreier JP, Farkas E. Malignant astrocyte swelling and impaired glutamate clearance drive the expansion of injurious spreading depolarization foci. J Cereb Blood Flow Metab. 2022 Apr;42(4):584-599. doi: 10.1177/0271678X211040056. (IF2022: 6,3) D1
-Molnár K, Mészáros Á, Fazakas C, Kozma M, Győri F, Reisz Z, Tiszlavicz L, Farkas AE, Nyúl-Tóth Á, Haskó J, Krizbai IA*, Wilhelm I*. Pericyte-secreted IGF2 promotes breast cancer brain metastasis formation. Mol Oncol. 2020 Sep;14(9):2040-2057. doi: 10.1002/1878-0261.12752. (IF2020: 6,603). D1. *levelező szerzők
-Haskó J, Fazakas C, Molnár K, Mészáros Á, Patai R, Szabó G, Erdélyi F, Nyúl-Tóth Á, Győri F, Kozma M, Farkas AE, Krizbai IA*, Wilhelm I*. Response of the neurovascular unit to brain metastatic breast cancer cells. Acta Neuropathol Commun. 2019 Aug 19;7(1):133. doi: 10.1186/s40478-019-0788-1 (IF2019: 6.270). D1. *levelező szerzők
-Hildegard Herman, Csilla Fazakas, János Haskó, Kinga Molnár, Ádám Mészáros, Ádám Nyúl-Tóth, Gábor Szabó, Ferenc Erdélyi, Aurel Ardelean, *Anca Hermenean, *István A. Krizbai, *Imola Wilhelm. Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J Cell Mol Med. 2019 Apr;23(4):2619-2631. doi: 10.1111/jcmm.14156 (IF2019: 4,486). Q1. *levelező szerzők
-Costea L, Mészáros Á, Bauer H, Bauer HC, Traweger A, Wilhelm I, Farkas AE*, Krizbai IA*. The Blood-Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int J Mol Sci. 2019 Nov 3;20(21):5472. doi: 10.3390/ijms20215472. (IF2019: 4,556). Q1. *levelező szerzők
-Wilhelm I, Fazakas C, Molnár K, Végh AG, Haskó J, Krizbai IA. Foe or friend? Janus-faces of the neurovascular unit in the formation of brain metastases. J Cereb Blood Flow Metab. 2018 Apr;38(4):563-587. doi: 10.1177/0271678X17732025. (IF2018: 6,040). D1.
-Menyhárt Á*, Farkas AE*, Varga DP, Frank R, Tóth R, Bálint AR, Makra P, Dreier JP, Bari F, Krizbai IA, Farkas E. Large-conductance Ca2+-activated potassium channels are potently involved in the inverse neurovascular response to spreading depolarization. Neurobiol Dis. 2018 Nov;119:41-52. doi: 10.1016/j.nbd.2018.07.026 (IF2018: 5,160). D1. *első szerzők
-Nyúl-Tóth Á, Kozma M, Nagyőszi P, Nagy K, Fazakas C, Haskó J, Molnár K, Farkas AE, Végh AG, Váró G, Galajda P, Wilhelm I, Krizbai IA. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav Immun. 2017 Aug;64:220-231. doi: 10.1016/j.bbi.2017.04.010. (IF2017: 6,306). D1

scientific adviser

senior research associate

senior research associate

research associate

research associate

research associate

research associate

junior research associate

junior research associate

PhD student

PhD student

PhD student

Szent-Györgyi student

Szent-Györgyi student
 István KRIZBAI
István KRIZBAI
|
scientific adviser | publications | CV |
 Imola WILHELM
Imola WILHELM
|
senior research associate | publications | CV |
 Attila FARKAS
Attila FARKAS
|
senior research associate | publications | CV |
 Csilla FAZAKAS
Csilla FAZAKAS
|
research associate | publications | CV |
 Ádám NYÚL-TÓTH
Ádám NYÚL-TÓTH
|
research associate | publications | CV |
 János HASKÓ
János HASKÓ
|
research associate | publications | CV |
 Mónika KRECSMARIK
Mónika KRECSMARIK
|
research associate | publications | CV |
 Fanni GYŐRI
Fanni GYŐRI
|
junior research associate | publications | CV |
 Tamás DUDÁS
Tamás DUDÁS
|
junior research associate | publications | CV |
 Tejal SHREEYA
Tejal SHREEYA
|
PhD student | publications | CV |
 Maryam NAEEM
Maryam NAEEM
|
PhD student | CV | |
 Valentina NAGY
Valentina NAGY
|
PhD student | publications | CV |
 Adél LÜVI
Adél LÜVI
|
Szent-Györgyi student | CV | |
 István TÓTH
István TÓTH
|
Szent-Györgyi student | CV |