
Research
1) Modeling biological barriers with chip devices
The main focus of our research group is the investigation of biological barriers ̶ such as the gut, lung and corneal epithelial barriers and the endothelial blood-brain barrier (BBB) ̶ using cell culture models. In our experiments complex co-culture models from multiple cell types are used (Veszelka et al., 2018) to better mimick organs and organ systems (Figure 1). Our aim is to establish a new generation of barrier culture models using human stem cells as well as microfluidic and microelectronic chip devices to investigate disease patomechanisms and drug delivery across biological barriers.
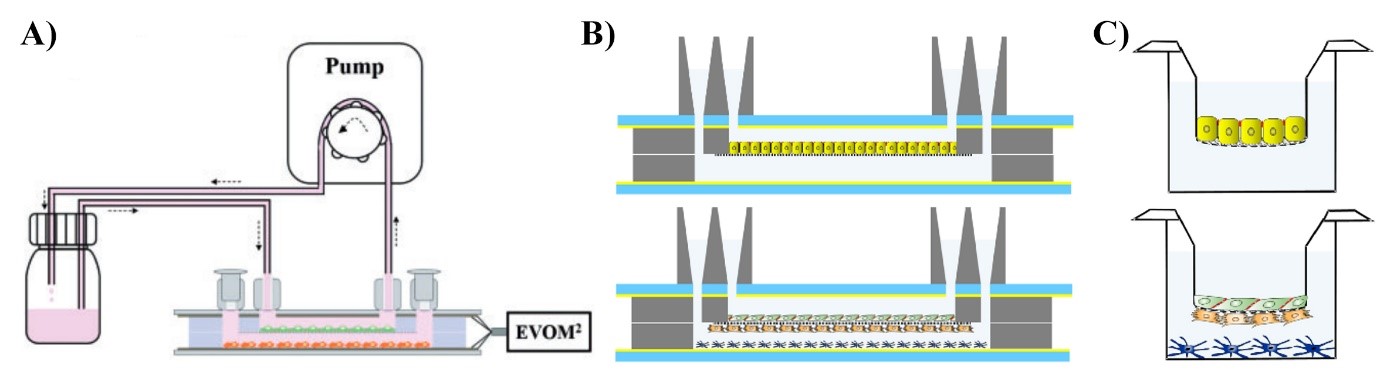
Figure 1. Tools to model biological barriers under dynamic (A, B) and static (C) conditions. Cell layers cultured in chip tools (B) and on cell culture inserts (C) separate two liquid containing compartments. The microfluidic biochip device enables the induction of fluid flow, which mimicks blood flow, (A) to create physiologically more relevant models.
We use static, cell culture insert-based, as well as dynamic, biochip-based models developed by our team (Walter et al., 2016; Kincses et al., 2020; Santa-Maria et al., 2021; Figure 1). These devices are cutting edge tools to study biological barriers and enable us to create a solid basis for further innovative and internationally competitive research and drug development.
2) Improving drug delivery across biological barriers
Biological barriers, including the epithelium of the intestinal and respiratory systems and the endothelium lining the inner surface of blood vessels, protect organisms from damaging agents and create homeostasis for physiological functions. These barriers, however, also hinder drug penetration and thereby the effective treatment of several diseases. Our team has developed nanoparticles functionalized with multiple ligands targeting BBB transporters that enhance drug penetration. Our targeted nanoparticles enhance drug delivery not only across the brain endothelial cell layer, but also into other brain cell types (Porkoláb et al., 2020, Figure 2) and into stem cell derived brain organoids (Mészáros et al., 2023).
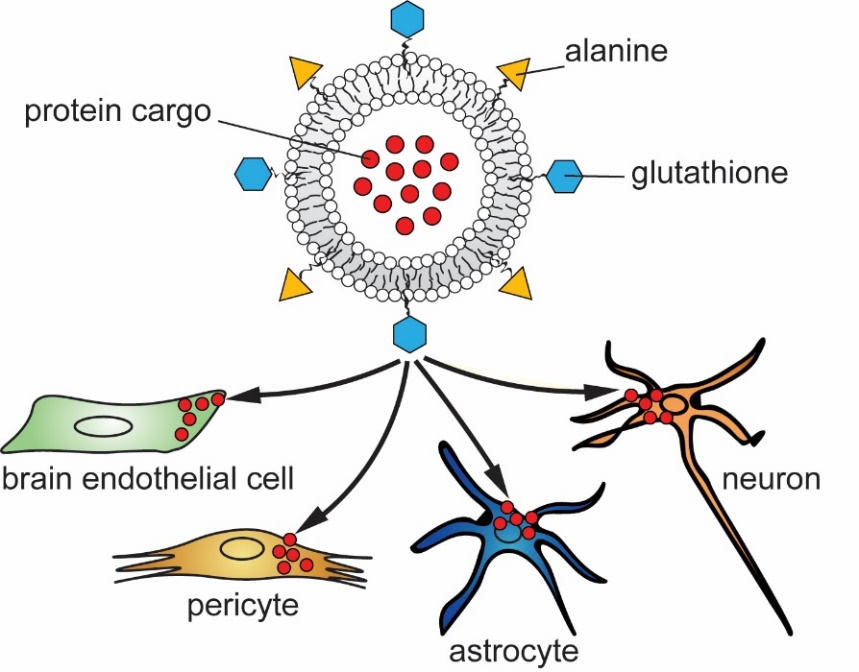
Figure 2. Nanoparticles targeting nutrient transporters of brain endothelial cells can enhance drug delivery across the blood-brain barrier and into pericytes, astrocytes and neurons.
3) Dysfunction of biological barriers and their protection
As the main role of biological barriers is to protect certain organs, their dysfunction in different diseases can have serious health consequences. BBB damage is a common feature of central nervous system pathologies that either causes the disease or worsens its progress. Our goal is to identify key factors in the development of diseases that effect barrier functions and to find potential compounds to protect or restore the integrity of barriers. In recent years we have investigated pathomechanisms and protection strategies in several neuropathological processes, such as neuroinflammation induced by kainic acid (Barna et al., 2020) and cytokines (Barabási et al., 2023), and BBB damage induced by acute pancreatitis (Walter et al, 2023).
Selected publications
Barabási B, Barna L, Santa-Maria AR, Harazin A, Molnár R, Kincses A, Vigh JP, Dukay B, Sántha M, Tóth ME, Walter FR, Deli MA, Hoyk Z. Role of interleukin-6 and interleukin-10 in morphological and functional changes of the blood-brain barrier in hypertriglyceridemia. Fluids Barriers CNS. 2023, 20:15.
Barna L, Walter FR, Harazin A, et al. Simvastatin, edaravone and dexamethasone protect against kainate-induced brain endothelial cell damage. Fluids Barriers CNS. 2020;17:5.
Kincses A, Santa-Maria AR, Walter FR, Dér L, Horányi N, Lipka DV, Valkai S, Deli MA, Dér A. A chip device to determine surface charge properties of confluent cell monolayers by measuring streaming potential. Lab Chip. 2020;20:3792-3805.
Mészáros M, Phan THM, Vigh JP, Porkoláb G, Kocsis A, Páli EK, Polgár TF, Walter FR, Bolognin S, Schwamborn JC, Jan JS, Deli MA, Veszelka S. Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood-Brain Barrier Model and Entry to Human Brain Organoids. Cells. 2023,12:503
Porkoláb G, Mészáros M, Tóth A, Szecskó A, Harazin A, Szegletes Z, Ferenc G, Blastyák A, Mátés L, Rákhely G, Deli MA, Veszelka S. Combination of alanine and glutathione as targeting ligands of nanoparticles enhances cargo delivery into the cells of the neurovascular unit. Pharmaceutics. 2020;12:635.
Santa-Maria AR, Walter FR, Figueiredo R, Kincses A, Vigh JP, Heymans M, Culot M, Winter P, Gosselet F, Dér A, Deli MA. Flow induces barrier and glycocalyx-related genes and negative surface charge in a lab-on-a-chip human blood-brain barrier model. J Cereb Blood Flow Metab. 2021, 41:2201-2215.
Veszelka S, Tóth A, Walter FR,Tóth AE, Gróf I, Mészáros M, Bocsik A, Hellinger É, Vastag M, Rákhely G, Deli MA. Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model With Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport. Front Mol Neurosci. 2018,11:166.
Walter FR, Harazin A, Tóth AE, Veszelka S, Santa-Maria AR, Barna L, Kincses A, Biczó G, Balla Z, Kui B, Maléth J, Cervenak L, Tubak V, Kittel Á, Rakonczay Z Jr, Deli MA. Blood-brain barrier dysfunction in L-ornithine induced acute pancreatitis in rats and the direct effect of L-ornithine on cultured brain endothelial cells. Fluids Barriers CNS. 2022, 19:16.

research professor

senior research associate

research associate

research associate

research associate

research associate

research associate

research associate

PhD student

PhD student

PhD student

Szent-Györgyi student
 Mária DELI
Mária DELI
|
research professor | publications | CV |
 Szilvia VESZELKA
Szilvia VESZELKA
|
senior research associate | publications | CV |
 Zsófia HOYK
Zsófia HOYK
|
research associate | publications | CV |
 Fruzsina WALTER
Fruzsina WALTER
|
research associate | publications | CV |
 Alexandra BOCSIK
Alexandra BOCSIK
|
research associate | publications | CV |
 András HARAZIN
András HARAZIN
|
research associate | publications | CV |
 Mária MÉSZÁROS
Mária MÉSZÁROS
|
research associate | publications | CV |
 Ilona GRÓF
Ilona GRÓF
|
research associate | publications | CV |
 Judit VIGH
Judit VIGH
|
PhD student | publications | CV |
 Gergő PORKOLÁB
Gergő PORKOLÁB
|
PhD student | publications | CV |
 Anikó SZECSKÓ
Anikó SZECSKÓ
|
PhD student | publications | CV |
 Lucien LEMAITRE
Lucien LEMAITRE
|
Szent-Györgyi student | publications | CV |