
I. Establishment of entirely green renewable energy production systems
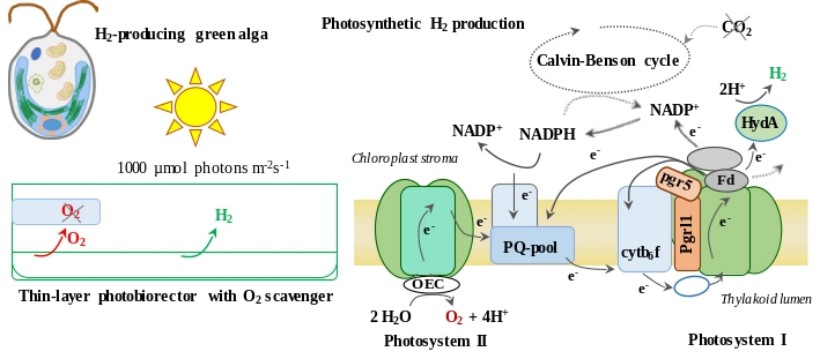
Water-splitting-based, sustainable, and efficient H2 production in green algae
Hydrogen gas (H2) is a clean energy carrier that features zero carbon emissions during its combustion. Green algae are capable of generating H2 during photosynthesis by their highly active [Fe-Fe]-type hydrogenases; for this reason, the photoproduction of H2 could theoretically be the most efficient way of converting sunlight into chemical energy by biological systems.
We have established a novel H2 production method by green algae that is based on a short anaerobic induction, keeping the Calvin–Benson cycle inactive by substrate limitation and preserving hydrogenase activity by applying a simple absorbent to remove the evolved O2. Cultures remained photosynthetically active for several days, with the electrons feeding the hydrogenases mostly derived from water. The amount of H2 produced is remarkable and the process is photoautotrophic. Our protocol demonstrated that it is possible to sustainably use algal cells as whole-cell catalysts for H2 production, which enables industrial application of algal biohydrogen production (Nagy V and Tóth SZ (2017) European Patent EPA 17155168.2; Nagy et al., 2018, Biotechnol Biofuels 11: 69).
In a follow-up study, we employed thin layer cultures, resulting in a three-fold increase in H2 production relative to bulk cultures. Productivity was maintained even at the intensity of sunlight. Remarkably, the L159I-N230Y photosystem II mutant and the pgrl1 photosystem I cyclic electron transport mutant produced 50% more H2 than the wild-type, while the pgr5 mutant generated 250% more (1.2 ml H2/ml culture in six days). The photosynthetic apparatus and the hydrogenase of the pgr5 mutant remained remarkably stable (Nagy et al., 2021 Biores. Technol. 333: 125217).
We are currently investigating the effects of varying light intensities and aim at upscaling our anaerobiosis-based photoautotrophic H2 production system.
Establishing efficient alga based electric current production systems
Promising biology-based alternatives to fossil fuels are the production of biohydrogen and photocurrent by so-called bio-photovoltaic (BPV) devices, also known as bio-photo electrochemical cells. BPV devices can convert the energy of sunlight, captured by photosynthetic organisms, into electric current. The electron flow generated by photosynthesis is transferred directly or indirectly via an electron mediator to the working electrode. Particularly suitable systems are the ones based on cyanobacteria and green algae. However, the currently available algae-based BPV systems have low industrial value, due to the low efficiency, limited energy producing capacity and reliance on harmful chemicals in the production process
In the frame of a Marie Skłodowska-Curie Actions project (EnergUP, Project Number: 892632), we aim at improving the performance of algae-based BPV devices by better understanding its limitations and try to overcome of those.
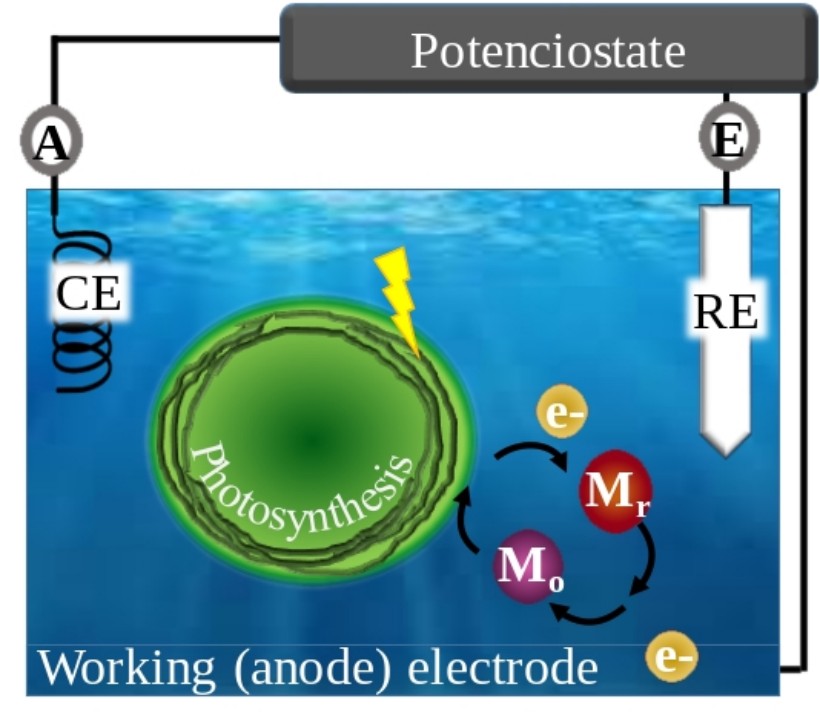
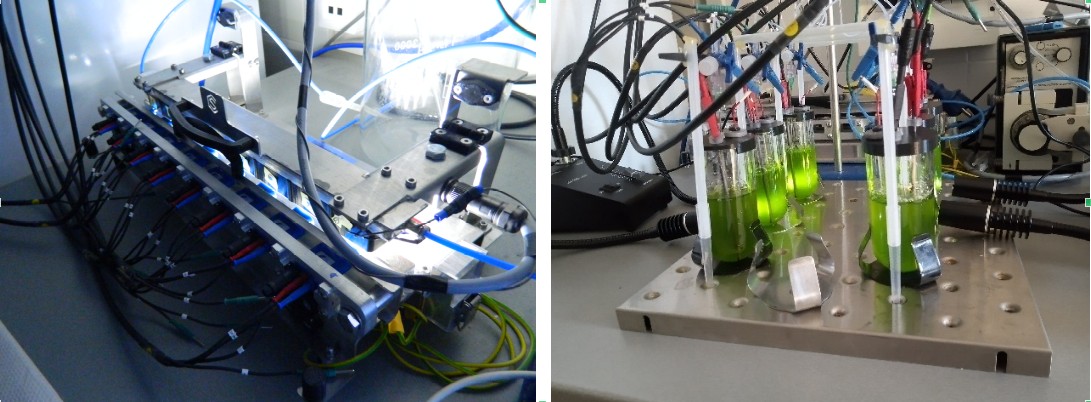
To this end, we have designed and built two bio-photovoltaic instruments suitable for measuring current production by algae samples in small (µl) or larger (tens of ml) volumes on a short (min) or a long (days) time-scale.
Our small BPV instrument enables the parallel measurement of up to eight alga samples at different light intensities. The sample holders accommodate commercially available screen printed electrodes. By the application of the small-volume BPV device, we have screened several mutant lines of Chlamydomonas for enhanced current production in order to identify potential target points in the photosynthetic and respiratory electron transport chains to enhance electric current production. In the best performing mutants, we obtained a 5- to 10-fold increase in the maximal current production capacity relative to wild-type strains. In addition, we have also tested several green algal species and found one with a great potential to be used in BPV devices.
Our newly built large-volume tube BPV device enables current production for several days. The altered orientation and surface ratios of the electrodes resulted in high current production in dilute cell cultures: The current normalized to the working electrode area and the chlorophyll content of the culture was about 200-fold higher in the large-volume BPEC device than in the small-volume BPV device.
 |
II. The roles of ascorbate (vitamin C) in plants and green algae
Ascorbate is an essential vitamin for humans, which is of plant origin and fulfills various roles both in mammalian and plant cells. The best-known property of ascorbate is to scavenge reactive oxygen species, in a highly regulated manner. Szilvia Z. Tóth and her group focus on the biosynthesis, transport and physiological roles of ascorbate in plants and green algae. Their results may ultimately contribute to increasing the ascorbate contents of crops.
In a recent review (Tóth SZ, 2023, Int J Mol Sci 24: 2537), the intricate relationship between ascorbate and photosynthesis in plants and algae was summarized in the following major points: (i) regulation of ascorbate biosynthesis by light, (ii) interaction between photosynthetic and mitochondrial electron transport in relation to ascorbate biosynthesis, (iii) ascorbate acting as an alternative electron donor of photosystem II, (iv) ascorbate inactivating the oxygen-evolving complex, (v) the role of ascorbate in non-photochemical quenching, and (vi) the role of ascorbate in oxidative stress management in the chloroplast. The review also discusses differences in the regulation of ascorbate biosynthesis and the effects of ascorbate on photosynthesis in algae and vascular plants.
Ascorbate may inactivate the oxygen-evolving complex
One of the earliest effects of prolonged darkness in plants is the inactivation of oxygen‐evolving complexes (OEC). We found that ascorbate inactivates the OEC in prolonged darkness by over‐reducing its manganese‐complex that is probably enabled by a dark‐induced dissociation of the extrinsic OEC subunits (Podmaniczki et al., (2021) Physiol Plantarum 171: 232-245). Our study is an example that ascorbate may negatively affect certain cellular processes and thus its concentration and localization need to be highly controlled.

The role of ascorbate in non-photochemical quenching in green algae
We demonstrated that, in contrast to seed plants, the violaxanthin de-epoxidase found in Chlamydomonas does not require ascorbate as a reductant. The rapidly induced, energy-dependent non-photochemical quenching component was not limited by ascorbate deficiency either. On the other hand, a reactive oxygen species-induced photoinhibitory quenching component was greatly enhanced upon ascorbate deficiency (Vidal-Meireles et al., 2020 Plant Physiol 182: 597–611). Our results demonstrate that ascorbate has distinct roles in non-photochemical quenching in Chlamydomonas as compared to vascular plants.
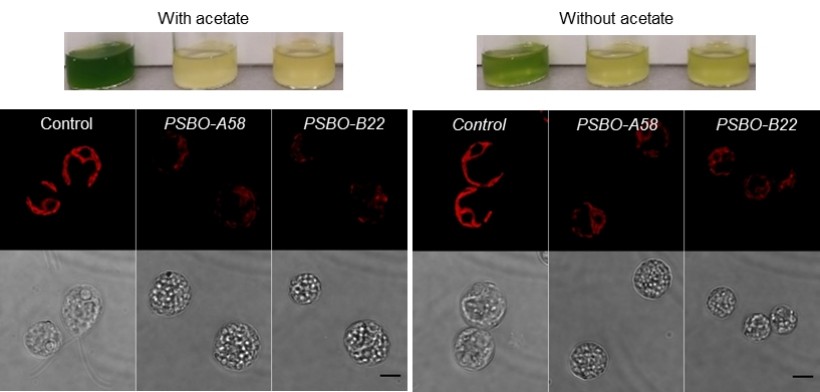
The lifetime of the oxygen-evolving complex subunit PSBO
PSBO is essential for the assembly of the oxygen-evolving complex but information on its lifetime was lacking in green algae. Using nitrate-inducible PSBO amiRNA lines in Chlamydomonas, we demonstrated that the lifetime of PSBO strongly depends on the light intensity and carbon availability, and thus, on the metabolic status of the cells. We also confirmed that PSBO is required for photosystem II stability in Chlamydomonas and demonstrate that its specific loss also entails substantial changes in cell morphology and cell cycle (Vidal-Meireles et al., 2023, Plant Cell Physiol 46: 422-439).
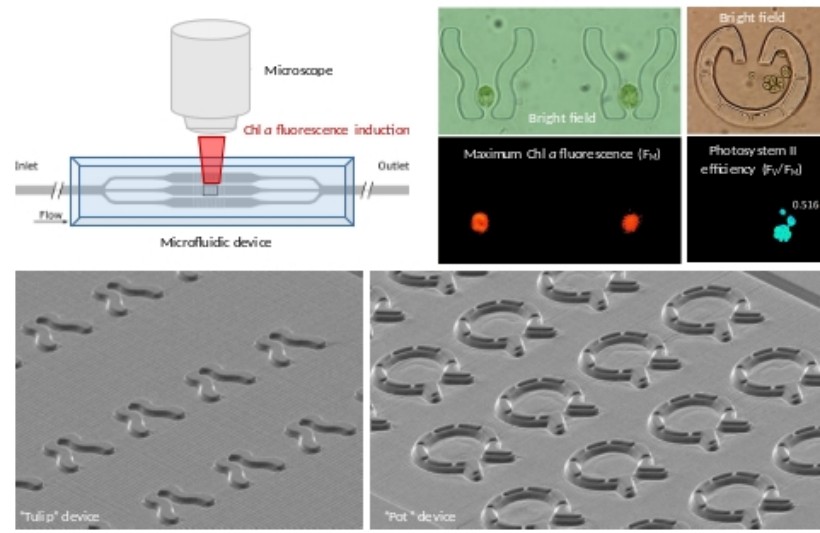
II. Microfluidics for morphological and photosynthetic investigations of Chlamydomonas on a single-cell level
We have established microfluidics in combination with chlorophyll a fluorescence induction measurements, in order to facilitate studying the relationship between morphology and photochemistry in Chlamydomonas on a single cell level. We developed two types of microfluidic platforms for single-cell investigations: (i) The traps of the “Tulip” device are suitable for capturing and immobilizing single cells, enabling the assessment of their photosynthesis for several hours without binding to a solid support surface. (ii) The traps of the “Pot” device were designed for capturing single cells and allowing the growth of the daughter cells within the traps. Our microfluidic devices represent versatile platforms for the simultaneous morphological and photosynthetic investigations of Chlamydomonas on a single-cell level (Széles et al., 2022, Cells 11: 285; Széles et al., 2023 Photosynthetica ps.2023.028).

scientific advisor

senior research associate

research associate

research associate

research associate

research assistant

junior research associate

technical assistant

laboratory assistant
 Szilvia Zita TÓTH
Szilvia Zita TÓTH
|
scientific advisor | publications | CV |
 Valéria NAGY
Valéria NAGY
|
senior research associate | publications | CV |
 Nia PETROVA ZLATKOVA
Nia PETROVA ZLATKOVA
|
research associate | publications | CV |
 Soujanya KUNTAM
Soujanya KUNTAM
|
research associate | publications | CV |
 Eszter SZÉLES
Eszter SZÉLES
|
research associate | publications | CV |
 László KOVÁCS
László KOVÁCS
|
research assistant | publications | CV |
 Dávid TÓTH
Dávid TÓTH
|
junior research associate | publications | CV |
 Éva HERMAN
Éva HERMAN
|
technical assistant | publications | CV |
 Zsombor DABOSI
Zsombor DABOSI
|
laboratory assistant | publications | CV |